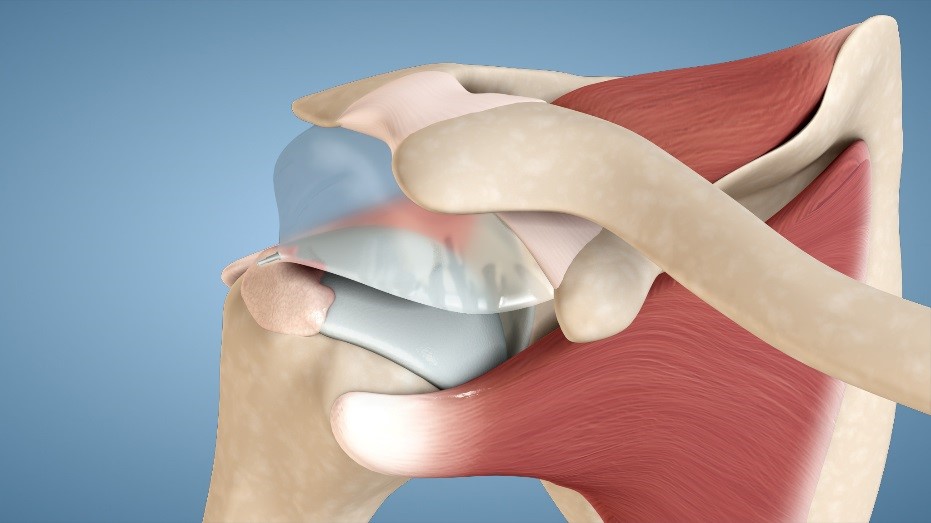
This ongoing clinical study of the InSpace System will ultimately enroll up to 45 patients, all of whom will be followed for up to 24 months. The study population consists of elderly patients who are not candidates for surgery under general anesthesia and who did not respond to conservative treatment, including steroid injections and physical therapy. This publication represents a planned interim analysis of the first 15 patients enrolled, who were considered evaluable upon reaching 12 months of follow-up. All 15 patients in this cohort experienced overall improvements in shoulder function, as measured by the Constant Score and the American Shoulder and Elbow Surgeons Evaluation Form (ASES). Of these patients, 85% showed a clinically significant improvement in their Constant Score. Improvements were observed beginning at 6 weeks post treatment and sustained for at least 12 months post-treatment. Additionally, patients reported significant improvements in pain scores.
“We are pleased with these results from Dr. Gervasi and colleagues. These data reinforce and continue to build on the existing data supporting the use of InSpace for patients with massive rotator cuff tears,” said Itay Barnea, CEO of OrthoSpace. “The cases represent an unmet clinical need, and we are proud to offer additional surgical options for patients who are suffering from pain and immobility associated with this difficult indication.”
About OrthoSpace Ltd.
OrthoSpace is a privately held medical device company located in Caesarea, Israel. The Company’s product, InSpace, is an orthopedic biodegradable balloon system that is simple, safe and a minimally invasive method that addresses unmet clinical needs in rotator cuff repair. InSpace is CE Marked and commercialized in Europe and Israel and has been granted an Investigational Device Exemption (IDE) to initiate a pivotal human clinical study of the InSpace System in the United States.