OVERLAND PARK, Kan., Dec 13, 2018 /PRNewswire/ — Overland Park Surgical Suites is a state of the art outpatient surgical center and is the first outpatient surgery center in the Midwest to offer patients a robotics-assisted alternative to traditional knee replacement surgery.
“We were eager to bring the NAVIO robotics-assisted surgery to the area,” said Scott Abraham, M.D., Orthopedic Surgeon and founder of Overland Park Surgical Suites, LLC. “This exacting technique is the preferred approach for most patients and we’ve seen dramatic surgical outcomes.”
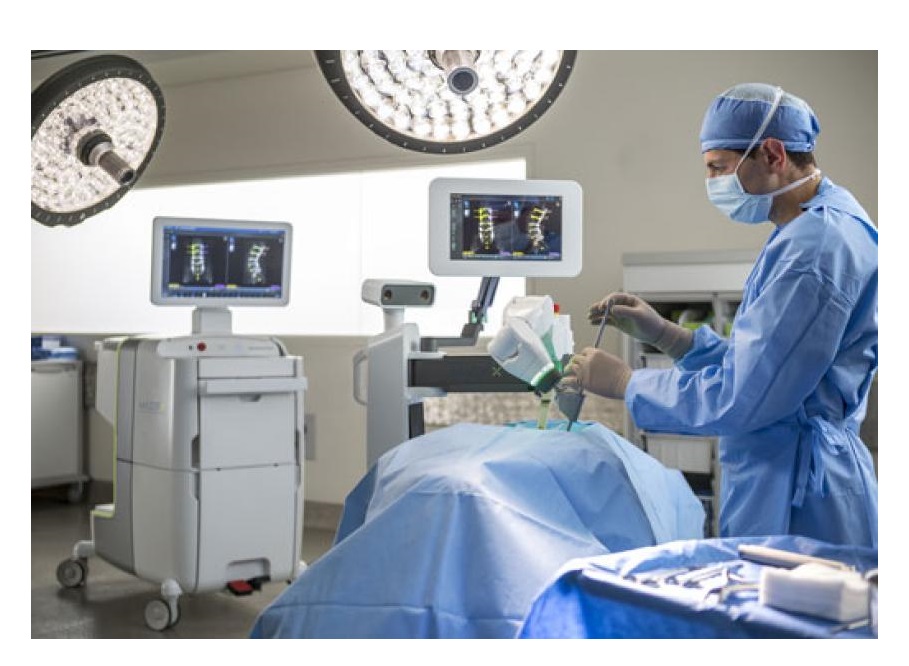
 The NAVIO Surgical System starts with an advanced computer system that gathers precise anatomic and alignment information about a damaged knee that a surgeon uses to create a specific surgical plan, according to Dr. Abraham.
The NAVIO Surgical System starts with an advanced computer system that gathers precise anatomic and alignment information about a damaged knee that a surgeon uses to create a specific surgical plan, according to Dr. Abraham.
“This extra layer of data collection is designed to ensure the knee procedure is performed as accurately as possible for the best long-term outcome,” he said.
Abraham has already performed more than 100 robotic-assisted partial and total knee replacements at Overland Park Surgical Suites and St. Joseph Medical Center, and draws patients to his practice from throughout Kansas, Missouri and Iowa. He said the NAVIO System is particularly well suited for active adults in their 40s and 50s who have developed knee problems, but have opted to put off surgery as a remedy.
“Knee replacement surgery has a controversial reputation,” Abraham added.
Unfortunately, this leads many patients to delay medical intervention because of concerns about invasive surgery, painful rehab and significant downtime. The NAVIO System helps offset these concerns with the improved accuracy and the potential for faster recovery, leading to the goal of improved surgical outcomes and increased patient satisfaction.
The first knee implant procedure was performed 50 years ago in 1968, according to the American Academy of Orthopaedic Surgeons. Now, more than 600,000 knee replacement surgeries are performed each year, making it one of the most in-demand surgical specialties in the country. Yet often, patients are dissatisfied with the outcome.
Not so for Beth Archer of Leawood, who in October was the first person in the state of Kansas to undergo a partial knee replacement using robotic surgical assistance at Overland Park Surgical Suites.
“I’d been living with knee pain for four years,” Archer said. “The quality of my life dramatically improved with my partial knee replacement done with the NAVIO Surgical System. I returned home immediately and was self-sufficient, had little pain and within a week I was walking without a cane or walker. I highly recommend this robotic surgery.”
Abraham added that the NAVIO benefits are many, yet robotic surgical-assist is still in its infancy.
“We’re proud that Overland Park Surgical Suites is the first outpatient surgery center in the region to offer this state of the art NAVIO knee replacement procedure,” Abraham said. “But we won’t be the last. In fact, we think this is the future of the surgical field. It puts the power of robotics in the skilled hand of the surgeon. And that means a better outcome for all our patients.”
For more information or to schedule a NAVIO consultation with Dr. Abraham, call APEX ORTHOPEDICS and Sports Medicine at 913-642-0200.
NOTE: Not all patients are candidates for surgery using the NAVIO Surgical System. Discuss your condition and implant options with your surgeon. Individual results of joint replacement vary. Implants may not produce the same feel or function as your original knee. There are potential risks with knee replacement surgery such as loosening, fracture, dislocation, wear and infection that may result in the need for additional surgery. NAVIO is not for everyone. Children, pregnant women, patients who have mental or neuromuscular disorders that do not allow control of the knee joint, and morbidly obese patients should not undergo a NAVIO procedure.
(www.OverlandParkSS.com)
Overland Park Surgical Suites is a state-of-the-art, orthopedic outpatient surgical center in Overland Park, KS. It was established by clinical personnel to offer safe, high quality surgical care. It is one of the first surgery centers in the Kansas City area to perform outpatient total joint replacements and is licensed by the State of Kansas and accredited by The Joint Commission.
About Smith & Nephew:
(www.RediscoverYourGo.com)
Smith & Nephew is a global medical technology business dedicated to helping healthcare professionals improve people’s lives. With leadership positions in Orthopaedic Reconstruction, Advanced Wound Management, Sports Medicine and Trauma & Extremities, Smith & Nephew has around 15,000 employees and a presence in more than 100 countries. Annual sales in 2017 were more than $4.8 billion. Smith & Nephew is a member of the FTSE100.
TM is a Trademark of Smith & Nephew
|
Contact: |
Betsy Donnelly |
|
Phone: |
913 208 5400 |
|
Email: |
SOURCE Overland Park Surgical Suites