Moximed: First Patient Treated in US Study of Atlas® System for Unicompartmental Knee Osteoarthritis
November 29, 2016
HAYWARD, Calif.–(BUSINESS WIRE)–Moximed®, Inc. announced today that the first patient has been treated in an FDA-approved IDE study for its latest generation unicompartmental unloading implant, the Atlas System. The Atlas System builds on the company’s eight years of clinical success with joint unloading implants for medial knee osteoarthritis (OA).
“I’m pleased to treat the first patient in the Atlas IDE study. I see in my practice a tremendous number of younger, early OA patients who are seeking an alternative to arthroplasty that will allow them to maintain a highly active lifestyle,” noted Andreas Gomoll, M.D., Associate Professor of Orthopaedic Surgery at Harvard Medical School and Director of the Orthopaedic Program at the Brigham and Women’s Hospital Center for Regenerative Medicine. “Load distribution plays an important role in early osteoarthritis, and these patients could potentially benefit from a joint unloading procedure.”
The Principal US Investigator of the previous Moximed clinical study (KineSpring System), Jack Farr, M.D., Director of the OrthoIndy Cartilage Restoration Center of Indiana and the OrthoIndy Sports Medicine Fellowship Program remarked, “The Atlas System provides the same 30 lbs. of joint unloading with a significantly smaller implant and anatomically-guided surgical technique. Patients intuitively understand the concept of a shock absorber and are excited about a procedure that preserves their own anatomy without the bone cuts associated with joint replacement.” He went on to say, “I am looking forward to enrolling my first patients in the Atlas Study.” His site in Indianapolis, Indiana is now open and recruiting patients.
“Beginning a US study with the Atlas System is a noteworthy accomplishment for the company,” stated Moximed CEO Kevin Sidow. “Following on the maturing data from the pivotal study of our previous generation device, we are now excited to study the Atlas System for US patients in the clinical setting. We anticipate the Atlas IDE study will reproduce the positive outcomes and safety data presented from the first international studies of the device.”
About Osteoarthritis
Osteoarthritis (OA), the most common form of arthritis, is a degenerative disease affecting the hands, knees, hips, feet and spine. According to the Centers for Disease Control and Prevention (CDC), OA affects 27 million adults in the US. It is caused by changes in cartilage, the soft tissue that cushions and protects bone, leading to pain and changes in the shape of the joint. In knee OA, as the cartilage wears away, the bone ends may begin to rub against each other, causing severe pain. While often regarded as a disease of the elderly, there are more symptomatic knee OA patients under 65 years of age than over 65 years and an annual incidence of knee OA more than five times higher in individuals under 65 years of age than over 65 years.
About Moximed
Moximed, Inc. is dedicated to improving the standard of care for patients with osteoarthritis (OA). OA, the most common form of arthritis, leads to a breakdown of the joint’s cartilage and often results in joint pain and loss of motion. Moximed is supported by world-leading venture investors including New Enterprise Associates (NEA), Vertex Venture Holdings Ltd., Gilde Healthcare Partners, Morgenthaler Ventures, and GBS Venture Partners. More information can be found at www.moximed.com.
About the Atlas System
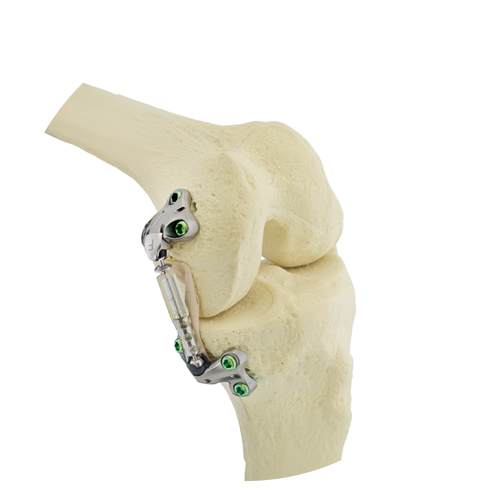
The Atlas System is designed as an implantable joint unloader for patients with medial knee osteoarthritis. Placed subcutaneously alongside the knee joint, the Atlas System incorporates advanced biomaterials designed to provide a clinically beneficial 30 lbs. of unloading. Importantly, the Atlas System absorbs excess joint load rather than transfer load to otherwise healthy areas of the joint. The Atlas System features a streamlined surgical technique that uses the patient’s own anatomy and allows the surgeon to visually confirm device function during the procedure. Finally, the Atlas System is joint preserving, and patients maintain all future treatment options should their osteoarthritis become more severe.
The Atlas System is CE Marked in Europe and is currently only being made available to select centers. The Atlas System is an investigational device in the United States, where it is limited by US law to investigational use.
Contacts
Moximed, Inc.
Keith Fong
Director of Marketing
Office: +1 510 887 3324
Cell: +1 650 245 4272
kfong@moximed.com
or
Halsin Partners
Mike Sinclair
Partner
Office: +44 (0)20 7318 2955
Cell: +44 (0)7968 022075
msinclair@halsin.com
OrthoSpineNews
OrthoSpineNews.com is the preferred aggregator of all news in the orthopedic and spine industry. You can subscribe for our daily email, follow us on twitter or download our app for iPhone and Android.