May 26, 2017
BREDA, Netherlands–(BUSINESS WIRE)–Freedom Neuro BV, a medical device distributor for Stimwave Technologies Incorporated, today announced CE Mark approval for the world’s first Percutaneous Injectable Anchor System. The injectable anchor is utilized in conjunction with the company’s wireless neurostimulator devices to provide true innovation in fixating Stimwave’s Wireless Pain Relief®technology through a minimally-invasive outpatient procedure for those who suffer from chronic pain.
“A wireless system that enables clinicians to actually have the full programming capabilities of IPGs, all in a device that can be injected, represents a paradigm shift in the field of options to provide the best in customization for patients to manage their pain profiles,” said Bart Billet, MD, from AZ Delta hospital group in Roeselare, Belgium.
Once the stimulator is injected, the SandShark is slid down over the device, progressing the un-deployed anchor into ligaments and strong connective tissues. Once the radiopaque anchor is in the desired location, the clinician pulls back on the handle, simultaneously deploying the wings of the anchor and pushing them laterally into the tissue. This injection process secures the anchor to the stimulator and secures the stimulator to the surrounding tissue to prevent migration throughout the life of the micro-implant. The injectable anchor stays in line with the body’s nerves, allowing for a freedom of movement generally not available in other neuromodulation devices.
“The SandShark Percutaneous Injectable Anchor System is a breakthrough for spinal cord stimulators, peripheral nerve stimulators, and all neurostimulation devices,” said Laura Tyler Perryman, founder and CEO of Stimwave. “It allows clinicians to inject a stimulator and its anchor through the same needle puncture port, opening the door for more clinicians to more easily provide patients with our highly efficient stimulators.”
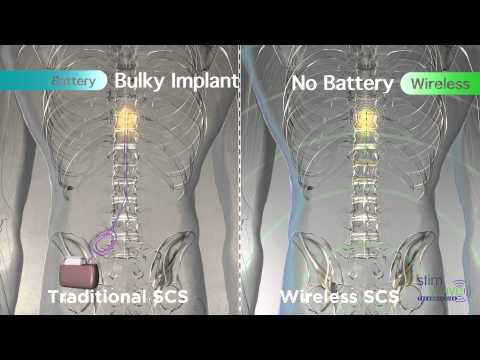
Aiming to provide a non-opioid alternative in the treatment of chronic pain, Stimwave has pioneered the Stimwave Freedom Spinal Cord Stimulation (SCS) System and the StimQ Peripheral Nerve Stimulator (PNS) System. Stimwave’s devices use Wireless Pain Relief®technology, are 95 percent smaller than any other neuromodulation device on the market and are the only system to have full body 3T MRI Conditionality. Representing a life-changing technological breakthrough for the more than 400 million people who endure daily chronic pain, the Stimwave Freedom Spinal Cord Stimulation (SCS) System is the most versatile system available in the industry. The CE Marked Freedom-8A SCS System can provide European patients with up to 64 electrode contacts and offer traditional programming options, as well as other programming options, including frequencies up to 10,000 Hz or waveform customization. The Freedom-8A SCS System with eight electrodes continues to utilize the Apple iPad programmer, leveraging Bluetooth protocols for ease of use in programming the variety of options. The devices deliver small pulses of energy to specific nerves, triggering a reaction that enables the brain to remap pain pathways, thus providing pain relief.
For more information, visit www.stimwave.com.
About Stimwave
Stimwave Technologies Incorporated is a privately held medical device company engaged in the development, manufacturing, and commercialization of wirelessly powered, microtechnology neurostimulators, providing patients with a convenient, safe, minimally invasive, and highly cost-effective pain management solution that is easily incorporated into their daily lives. Stimwave’s goal is to evolve its patented, cutting-edge platform into the default for neuromodulation, increasing the accessibility for patients worldwide while lowering the economic impact of pain management. www.stimwave.com
Statements made in this press release that look forward in time or that express beliefs, expectations or hopes regarding future occurrences or anticipated outcomes are forward-looking statements. A number of risks and uncertainties such as risks associated with product development and commercialization efforts, expected timing or results of any clinical trials, ultimate clinical outcome and perceived or actual advantages of the company’s products, market and physician acceptance of the products, intellectual property protection, and competitive offerings could cause actual events to adversely differ from the expectations indicated in these forward looking statements.
Contacts
Glodow Nead Communications
Evan Nicholson, Sonia Sparks, Kati Stadum, and Sarah Rogers, 415-394-6500
StimwavePR@GlodowNead.com