May 08, 2018
RESEARCH TRIANGLE PARK, N.C.–(BUSINESS WIRE)–TransEnterix, Inc. (NYSE American:TRXC), a medical device company that is digitizing the interface between the surgeon and the patient to improve minimally invasive surgery, today announced its operating and financial results for the first quarter 2018.
Recent Highlights
- During the first quarter ended March 31, 2018, the Company sold two Senhance Systems
- In January of 2018, the Company filed a FDA 510(k) submission to expand the indications for use of the Senhance System, potentially doubling the Senhance System’s total addressable procedures
- Thus far in the second quarter ending June 30, 2018, the Company has sold three Senhance Systems, including one in the U.S.
“We continued to generate momentum in the first quarter of 2018, including delivering the second consecutive quarter with multiple Senhance system sales and progressing our U.S. indication expansion strategy,” said Todd M. Pope, President and CEO at TransEnterix. “Looking to the balance of 2018, we will continue to leverage the momentum we have generated to drive the global commercial adoption of Senhance.”
Commercial and Clinical Update
In the quarter ended March 31, 2018, the Company sold two Senhance Systems. Both of these sales have come from sales to end user hospitals by distributors in the Company’s EMEA (Europe, Middle East, and Africa) region.
In January of 2018, the Company filed a 510(k) submission with the FDA to expand the indications for use of the Senhance System to include laparoscopic inguinal hernia and gallbladder surgery. The Senhance System is currently cleared for use in the U.S. for laparoscopic colorectal and laparoscopic gynecologic surgery, accounting for approximately 1.5 million procedures in the U.S. annually. Upon clearance, we anticipate these additional indications would bring the Senhance System’s total addressable procedures in the U.S. to approximately 3 million.
Thus far in the quarter ending June 30, 2018, the Company has sold three Senhance Systems. One of the system sales was in the U.S., driven by the Company’s direct sales force, the remaining two system sales came from sales to end user hospitals by distributors in the Company’s EMEA region.
First Quarter Financial Highlights
For the three months ended March 31, 2018, the Company reported revenue of $4.8 million as compared to revenue of $1.9 million in the three months ended March 31, 2017. Revenue in the first quarter of 2018 included $3.5 million in system sales, $1.1 million in instruments and accessories, and $200 thousand in services.
For the three months ended March 31, 2018, total net operating income and expenses were $5.4 million, as compared to $16.5 million in the three months ended March 31, 2017.
For the three months ended March 31, 2018, net loss was $0.9 million, or $0.00 per share, as compared to a net loss of $15.4 million, or $0.13 per share, in the three months ended March 31, 2017.
For the three months ended March 31, 2018, adjusted net loss was $11.3 million, or $0.06 per share, as compared to an adjusted net loss of $12.6 million, or $0.11 per share in the three months ended March 31, 2017, after adjusting for the gain from the sale of SurgiBot assets and non-cash charges for amortization of intangible assets, change in fair value of contingent consideration, and change in fair value of warrant liabilities.
Conference Call
TransEnterix, Inc. will host a conference call on Tuesday, May 8, 2018 at 4:30 PM ET to discuss its first quarter 2018 operating and financial results. To listen to the conference call on your telephone, please dial (844) 804-5261 for domestic callers or (612) 979-9885 for international callers and reference conference ID 4854118 approximately ten minutes prior to the start time. To access the live audio webcast or archived recording, use the following link http://ir.transenterix.com/events.cfm. The replay will be available on the Company’s website.
About TransEnterix
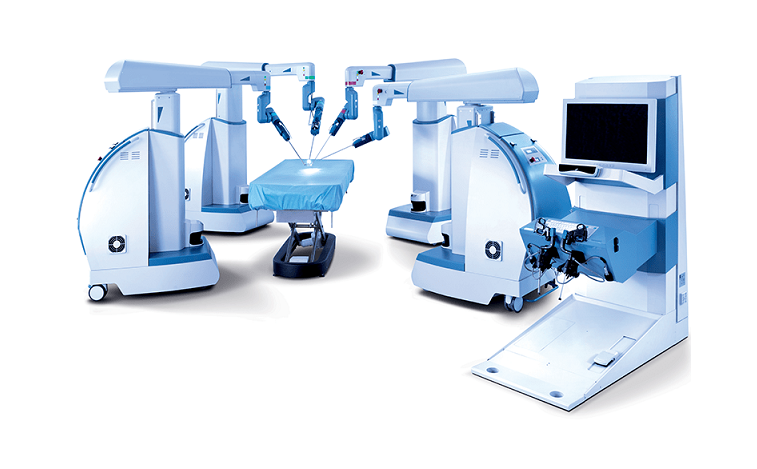
TransEnterix is a medical device company that is digitizing the interface between the surgeon and the patient to improve minimally invasive surgery by addressing the clinical and economic challenges associated with current laparoscopic and robotic options in today’s value-based healthcare environment. The Company is focused on the commercialization of the Senhance™ Surgical System, which digitizes laparoscopic minimally invasive surgery. The system allows for robotic precision, haptic feedback, surgeon camera control via eye sensing and improved ergonomics while offering responsible economics. The Senhance Surgical System is available for sale in the US, the EU and select other countries. For more information, visit www.transenterix.com.
Non-GAAP Measures
The adjusted net loss and adjusted net loss per share presented in this press release are non-GAAP measures. The adjustments relate to the gain on the sale of SurgiBot assets, amortization of intangible assets, change in fair value of contingent consideration and change in fair value of warrant liabilities. These financial measures are presented on a basis other than in accordance with U.S. generally accepted accounting principles (“Non-GAAP Measures”). In the tables that follow under “Reconciliation of Non-GAAP Measures,” we present adjusted net loss and adjusted net loss per share, reconciled to their comparable GAAP measures. These items are adjusted because they are not operational or because these charges are non-cash or non-recurring and management believes these adjustments are meaningful to understanding the Company’s performance during the periods presented. These Non-GAAP Measures should be considered a supplement to, not a substitute for, or superior to, the corresponding financial measures calculated in accordance with GAAP.
Forward-Looking Statements
This press release includes statements relating to the 2018 first quarter results and plans for 2018 and beyond. These statements and other statements regarding our future plans and goals constitute “forward looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, and are intended to qualify for the safe harbor from liability established by the Private Securities Litigation Reform Act of 1995. Such statements are subject to risks and uncertainties that are often difficult to predict, are beyond our control and which may cause results to differ materially from expectations and include whether the expansion of the indications for use of the Senhance System will be approved, whether upon clearance the Senhance System’s total addressable procedures in the U.S. will more than double to approximately three million procedures, and whether we will be able to leverage the momentum we have worked to generate to drive the global commercial adoption of Senhance. For a discussion of the risks and uncertainties associated with TransEnterix’s business, please review our filings with the Securities and Exchange Commission (SEC), including our Annual Report on Form 10-K for the year ended December 31, 2017, filed with the SEC on March 8, 2018 and our other filings we make with the SEC. You are cautioned not to place undue reliance on these forward looking statements, which are based on our expectations as of the date of this press release and speak only as of the origination date of this press release. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise.
|
TransEnterix, Inc. Consolidated Statements of Operations and Comprehensive Loss (in thousands except per share amounts) (Unaudited) |
||||||||
| Three Months Ended | ||||||||
| March 31, | ||||||||
| 2018 | 2017 | |||||||
| Revenue | $ | 4,767 | $ | 1,946 | ||||
| Cost of revenue | 2,555 | 1,334 | ||||||
|
Gross profit |
2,212 | 612 | ||||||
|
Operating Expenses (Income) |
||||||||
| Research and development | 5,265 | 6,855 | ||||||
| Sales and marketing | 5,970 | 3,723 | ||||||
| General and administrative | 2,676 | 3,049 | ||||||
| Amortization of intangible assets |
2,827 |
1,636 |
||||||
| Change in fair value of contingent consideration |
627 |
1,227 |
||||||
| Gain from sale of SurgiBot assets, net |
(11,996 |
) |
— |
|||||
|
Total Operating Expenses (Income) |
5,369 | 16,490 | ||||||
| Operating Loss | (3,157 | ) | (15,878 | ) | ||||
|
Other Income (Expense) |
||||||||
| Change in fair value of warrant liabilities |
1,829 |
— |
||||||
| Interest expense, net | (386 | ) | (334 | ) | ||||
|
Other expense |
(58 | ) | (60 | ) | ||||
|
Total Other Income (Expense), net |
1,385 | (394 | ) | |||||
| Loss before income taxes | $ | (1,772 | ) | $ | (16,272 | ) | ||
| Income tax benefit | 890 | 858 | ||||||
| Net loss | $ | (882 | ) | $ | (15,414 | ) | ||
|
Other comprehensive income |
||||||||
|
Foreign currency translation gain |
2,308 |
1,133 |
||||||
|
Comprehensive income (loss) |
$ | 1,426 | $ | (14,281 | ) | |||
| Net loss per share – basic and diluted |
$ |
0.00 |
$ |
(0.13 |
) |
|||
| Weighted average common shares outstanding – basic and diluted |
|
199,900 |
|
121,660 |
||||