This Press Release can be found at PRWeb.com and is also featured on other industry sites.
The Acquired Technology Will Complement Johnson & Johnson Medical Devices Companies’ Advanced Portfolio of Interbody Implants For Both Minimally Invasive and Open Spinal Fusion Surgery
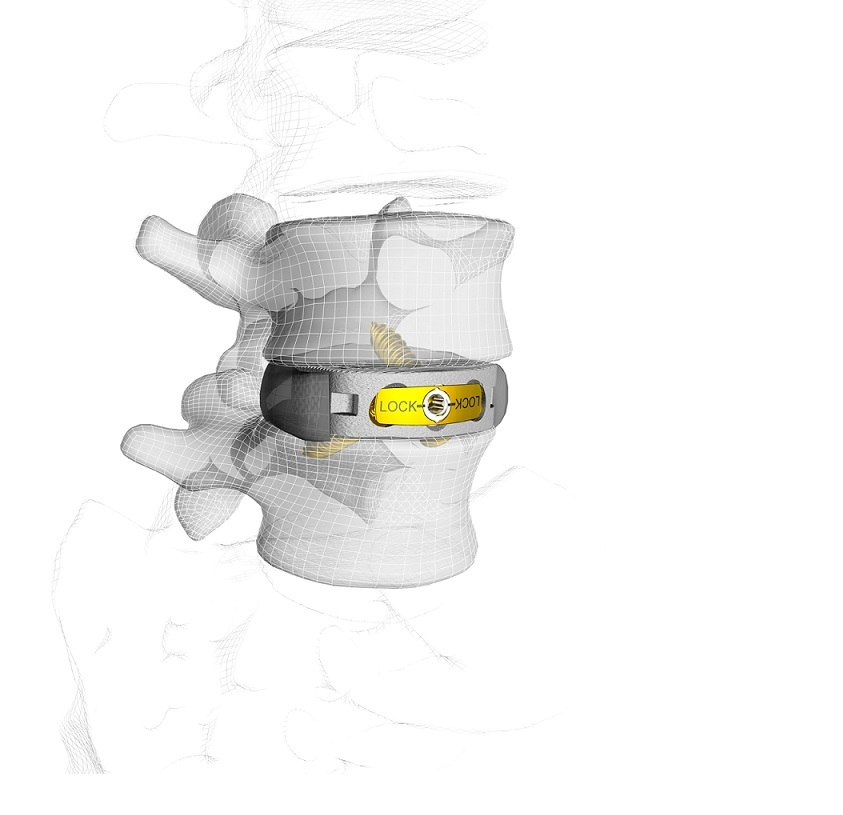
NORDERSTEDT, Germany, Sept. 12, 2018 /PRNewswire/ — Johnson & Johnson Medical Devices Companies*, through its subsidiary Johnson & Johnson Medical GmbH, announced today the acquisition of Emerging Implant Technologies GmbH (EIT), a privately held manufacturer of 3D-printed titanium interbody implants for spinal fusion surgery, based in Wurmlingen, Germany. The products in this portfolio leverage EIT’s proprietary advanced cellular titanium, which consists of an open and interconnected porous structure designed to allow bone to grow into the implant. As an industry leader across the full range of orthopaedic and spine specialties, Johnson & Johnson Medical Devices Companies will leverage its global commercial infrastructure to bring EIT’s technologies to patients around the world.
This acquisition allows DePuy Synthes, the orthopaedics business of Johnson & Johnson, to enhance its comprehensive interbody implant portfolio that includes expandable interbody devices, titanium integrated PEEK technology and now 3D-printed cellular titanium, for both minimally invasive and open spinal surgery. The EIT technology complements DePuy Synthes’ investment in the interbody implant segment in spine, including the recent introductions of the CONCORDE LIFT Expandable Interbody Device, and in the U.S., the PROTI 360°™ Family of Titanium-Integrated Interbody Implants, designed to treat patients with degenerative disc disease.
“Our goal is to offer a complete portfolio of interbody solutions that provides surgeons with even more options for the treatment of their patients,” said Aldo Denti, Company Group Chairman of DePuy Synthes. “We are excited to welcome the skilled team at EIT, and together, we aspire to bring to market technologies that allow surgeons to perform spinal fusion procedures reliably and with consistent outcomes.”
This acquisition underscores the companies’ commitment to building an innovative portfolio of spine solutions to improve the standard of care for patients. Moving forward, DePuy Synthes will continue to focus on the spinal disease states with the most potential for surgeons and their patients – degenerative disc disease, deformity and complex cervical – and introduce technologies in the fastest-growing segments within these disease states; specifically, interbody implants, enabling technologies, minimally invasive spine (MIS), and biomaterials.
Financial terms of the transaction will not be disclosed.
About DePuy SynthesDePuy Synthes, part of the Johnson & Johnson Medical Devices Companies, provides one of the most comprehensive orthopaedics portfolios in the world. DePuy Synthes solutions, in specialties including joint reconstruction, trauma, craniomaxillofacial, spinal surgery and sports medicine, are designed to advance patient care while delivering clinical and economic value to health care systems worldwide. For more information, visit www.depuysynthes.com.
About the Johnson & Johnson Medical Devices CompaniesAs the world’s most comprehensive medical devices business, we are building on a century of experience, leveraging science and technology, to shape the future of healthcare. With unparalleled breadth, depth and reach in surgery, orthopaedics and interventional solutions, we’re working to profoundly change the way care is delivered. We are in this for life. Learn more about our latest innovations by visiting: https://www.jnjmedicaldevices.com.
Cautions Concerning Forward-Looking StatementsThis press release contains “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 regarding the acquisition of EIT. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Johnson & Johnson Medical GmbH, any of the other Johnson & Johnson Medical Devices Companies and/or Johnson & Johnson. Risks and uncertainties include, but are not limited to: the potential that the expected benefits and opportunities of the acquisition may not be realized or may take longer to realize than expected; challenges inherent in product research and development, especially at an early stage of the development program, including the uncertainty of clinical success and obtaining regulatory approvals; uncertainty of commercial success for new products; manufacturing difficulties and delays; product efficacy or safety concerns resulting in product recalls or regulatory action; competition, including technological advances, new products and patents attained by competitors; challenges to patents; and changes to applicable laws and regulations, including tax laws and global health care reforms. In addition, there are risks and uncertainties related to the ability of the Johnson & Johnson family of companies to successfully integrate the technology, products, operations and employees of EIT, as well as the ability to ensure continued development, performance or market growth of EIT products. A further list and descriptions of these risks, uncertainties and other factors can be found in Johnson & Johnson’s Annual Report on Form 10-K for the fiscal year ended December 31, 2017, including in the sections captioned “Cautionary Note Regarding Forward-Looking Statements” and “Item 1A. Risk Factors,” and in the company’s most recently filed Quarterly Report on Form 10-Q, and the company’s subsequent filings with the Securities and Exchange Commission. Copies of these filings are available online at www.sec.gov, www.jnj.com or on request from Johnson & Johnson. Neither the Johnson & Johnson Medical Devices Companies nor Johnson & Johnson undertake to update any forward-looking statement as a result of new information or future events or developments.
*The Johnson & Johnson Medical Devices Companies comprise the surgery, orthopaedics, cardiovascular and specialty solutions businesses within Johnson & Johnson’s Medical Devices segment.
View original content to download multimedia:http://www.prnewswire.com/news-releases/johnson–johnson-medical-gmbh-acquires-emerging-implant-technologies-gmbh-to-enhance-global-offering-of-interbody-spine-implants-300710822.html
SOURCE Johnson & Johnson Medical Devices Companies
Copyright (C) 2018 PR Newswire. All rights reserved