MAYWOOD, IL – A surgery for quadriplegics called tendon transfer can significantly improve hand and elbow function, but the procedure is greatly underused, according to an article in the journal Hand Clinics by Loyola Medicine hand surgeon Michael S. Bednar, MD, FAAOS.
In the procedure, muscles that still work are redirected to do the jobs of muscles that are paralyzed. Depending on the extent of the spinal cord injury, tendon transfers can enable a patient to grasp objects, pinch, open the hand and straighten the elbow. The patient can, for example, propel a wheelchair in the snow, use a fork without splints, grip a fishing pole, shake hands and perform daily activities such as dressing, bathing, toileting and transferring to and from a wheelchair.
“Although the long-term outcomes of these procedures are good, few patients eligible for these procedures actually have them performed,” Dr. Bednar wrote.
Dr. Bednar has performed tendon transfers on about 60 patients, and is among the most skilled and experienced surgeons in the country doing the procedure. Dr. Bednar is a professor in the department of orthopaedic surgery and rehabilitation at Loyola University Chicago Stritch School of Medicine.
When quadriplegics were asked what function they would most like restored, 75 percent said hand function, followed in order by bowel and bladder use (13 percent), walking (8 percent) and sexual performance (3 percent), according to an earlier study cited in Dr. Bednar’s article. However, only 14 percent of patients who are surgical candidates wind up getting tendon transfers, according to another previous study.
Patients who stand to benefit most from tendon transfers have spinal cord injuries in the C5-C8 cervical nerves in the lower neck. Patients must not have acute or chronic medical conditions such as infections, pressure sores, medical instability or spasticity.
“A good surgical candidate has functional goals, is motivated, understands benefits and limitations of surgery, demonstrates emotional and psychological stability/adjustment to disability and is committed to the post-operative rehabilitation process,” Dr. Bednar wrote.
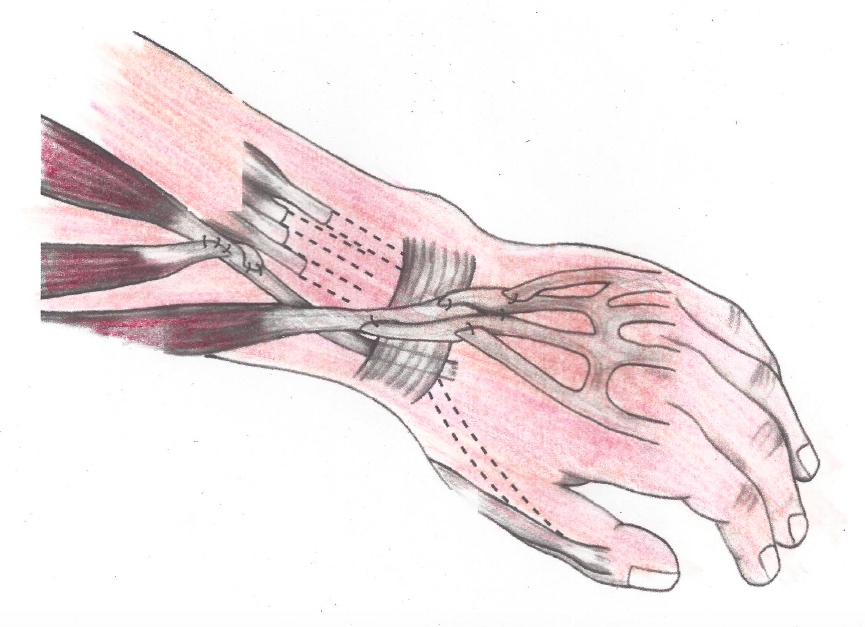
Skeletal muscles come in pairs – one muscle to move the bone in one direction, another muscle to move it back. Muscles are connected to bones by tendons. The bone moves when the brain sends a signal down a nerve telling the muscle to contract.
In many cases, more than one muscle performs the same function. So in a tendon transfer, the surgeon shifts the tendon of one of the spare muscles to a new location. For example, the surgeon may detach one of the working elbow muscles (the brachioradialis) and reattach it to a nonworking muscle that flexes the thumb (the flexor pollicis longus).
The number of functioning muscles a patient has will determine what tendon transfers the surgeon will perform. The more working muscles available for transfer, the more functions can be restored.
Tendon transfers typically involve two surgeries on each arm, performed three months apart. Arms are done one at a time. During rehabilitation, patients learn how to use the transferred muscles.
Tendon transfers temporarily reduce hand and elbow function during recovery and rehabilitation. Tendon transfers also do not restore full function. But while pinch strength and grasp strength after rehabilitation are not as high as in a normal hand, they are high enough to perform most activities of daily living.
Among the reasons so few patients get tendon transfers are lack of communication among rehabilitation specialists, physicians and surgeons, poor access to care and lack of awareness. The greatest barrier appears to be a lack of coordinated collaboration among specialists, Dr. Bednar wrote.
Dr. Bednar concluded: “Continued education of patients with tetraplegia, their caregivers and the rehabilitation community will hopefully increase utilization of these effective tendon transfer procedures.” (Tetraplegia is another term for quadriplegia.)
Dr. Bednar’s paper is titled “Tendon Transfers for Tetraplegia.”
– Loyola University Health System