August 29, 2016
MIAMI–(BUSINESS WIRE)–Stimwave LLC, a medical device manufacturer and independent research institute headquartered in South Florida, has achieved long-term success for hundreds of pain patients during the early adaptor rollout of the world’s first miniature wireless pain relief system. Embraced by pain specialists seeking non-opioid treatments, the drug-free, breakthrough remedy offers new hope to the more than 400 million people worldwide who suffer from chronic pain.
“Every day in the U.S. an average of 120 people die as a result of drug overdose, more than from motor vehicle crashes, and another 6,700-plus are treated in emergency departments for the misuse or abuse of prescription pain medication,” said Stimwave Chairman and CEO Laura Tyler Perryman. “We believe that Stimwave’s Wireless Pain Relief® technology is not only a viable option to help millions of people who suffer from chronic pain, but a potential life-saver as the U.S. faces an epidemic of opioid addiction that in many cases is an unavoidable side effect of long-term use of addictive pain medication.”
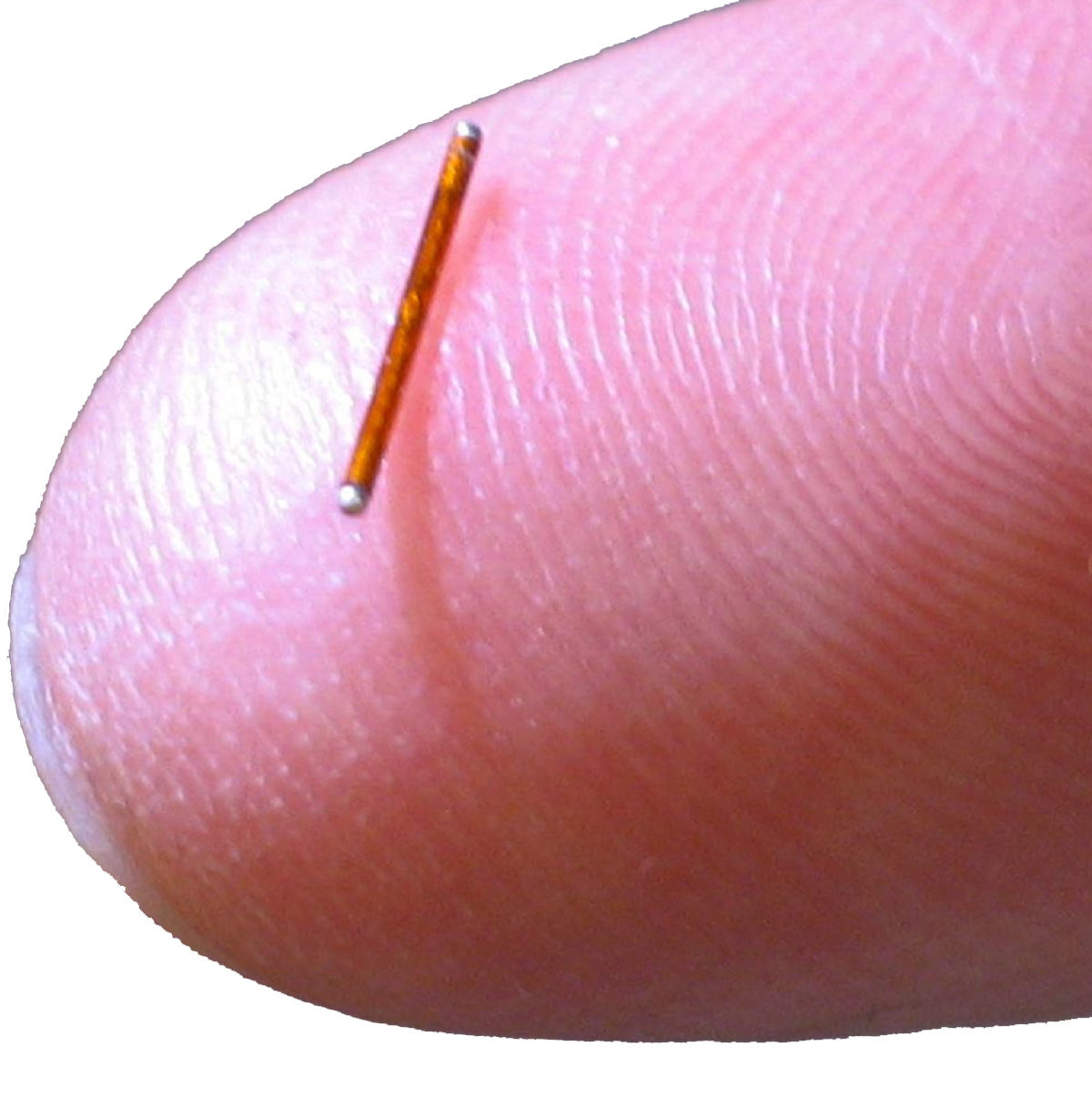
Stimwave’s Wireless Pain Relief devices are 95 percent smaller than any other neuromodulation device on the market, so small, with a diameter of less than 1.5 millimeters, that they are simply implanted through a standard needle during an outpatient procedure and a minimally invasive receiver placement technique. Because they are wireless, with the power source discreetly worn outside the body, there is no need for the invasive “open surgery” required of earlier neuromodulation devices, reducing the risk of infection, pain at the site of a large battery, and other long-term complications of battery units and connectors that account for 85 percent of adverse events, according to registries. Further, this miniature wireless device is powered by a wearable antenna, easily integrated into clothing and requiring no sticky gels that could irritate the skin, and there are no “wires” protruding from the body.
“This is great news for pain specialists and chronic pain patients who previously did not have a minimally-invasive implant option available for pain,” said Dr. Sanjay Gupta, president of the American Pain Association and principal clinician at Atlantic Pain and Wellness Institute. “Our country is facing a horrible epidemic of drug overdose deaths. These wireless products provide an alternative to opioids, which is much needed in the armamentarium for effective pain control.”
Stimwave launched it’s FDA-cleared devices for the relief of chronic back and leg pain to a limited number of patients throughout 2015, and in March 2016 was granted FDA 510(k) for the relief of peripheral nervous system (PNS) pain, becoming the only neuromodulation device manufacturer cleared by the FDA to help reduce chronic neuropathic pain at most locations throughout the body, from back and leg pain addressed by spinal cord stimulation to PNS treatment for shoulder pain, wrist and elbow pain, knee pain, hip pain and more.
“The major issue with peripheral nerve stimulators in the past has always been the bulk and length of cables, connectors and pulse generators,” said Dr. Richard North, consultant and retired Professor of Neurosurgery at John Hopkins University School of Medicine. “A miniature wireless peripheral nerve stimulator will minimize the need for surgery in patients who already are suffering from pain. It has long been needed and now is finally a reality.”
Stimwave’s wireless device delivers small pulses of energy to specific nerves, triggering a reaction that enables the brain to remap pain pathways, thus providing pain relief. The Stimwave device contains no internal batteries or other toxic materials. The device is fixed in place by an anchor, so it stays “in line” with the body’s nerves, allowing a freedom of motion that is impossible with bulkier implanted devices.
The Stimwave portfolio also includes the only neuromodulation system approved for “full body” MRI examinations, meaning the device does not have to be removed for 3-Tesla MRI exams. This is a significant diagnostic breakthrough as it allows patients with pain to benefit from neuromodulation without restricting their access to MRI-assisted diagnoses, for current or future medical issues.
“Stimwave saved my life. Without this amazing product, I would still be in terrible pain, on medication, and having my health fail,” said Anthony Torres, a cargo crane operator severely injured in an industrial accident. [Please see accompanying Patient Profiles.] “The external wireless batteries are small, effective, and simple-to-use.
“The best thing about Stimwave is that it gave me relief from the pain and allowed me to get off the pain medications. I wanted to get off all the pain medications.”
Please visit www.stimwave.com for more information.
About Stimwave
Stimwave Technologies Incorporated is a privately held medical device company engaged in the development, manufacture, and commercialization of wirelessly powered, microtechnology neurostimulators, providing patients with a convenient, safe, minimally invasive, and highly cost-effective pain management solution that is easily incorporated into their daily lives. Stimwave’s goal is to evolve its patented, cutting-edge platform into the default for neuromodulation, increasing the accessibility for patients worldwide while lowering the economic impact of pain management.
Contacts
Glodow Nead Communications
Casey Shaughnessy, Sonia Sparks and Evan Nicholson, 415-394-6500
stimwavepr@glodownead.com