GAINESVILLE, Fla.–(BUSINESS WIRE)– October 6, 2016
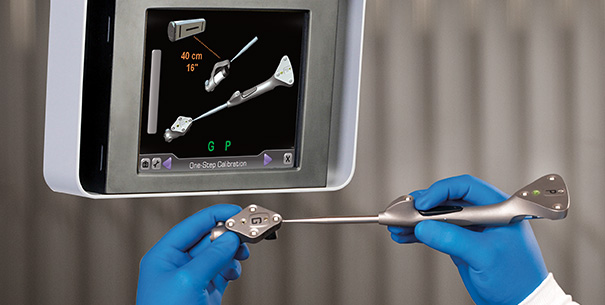
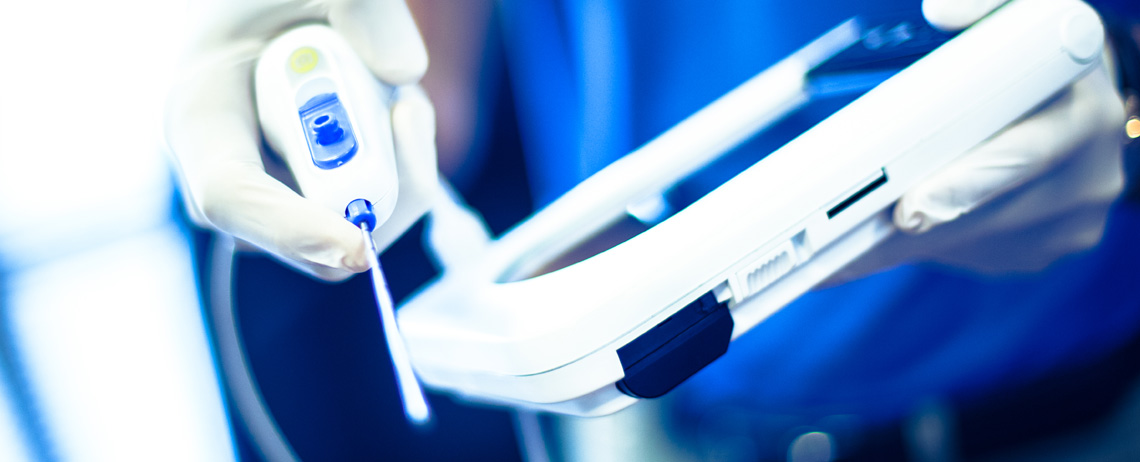
Exactech, Inc. (EXAC), a developer and producer of bone and joint restoration products for hip, knee, shoulder and spine, today announced today the successful first U.S. surgery using the new application of ExactechGPS® Guided Personalized Surgery system for revision knee procedures.
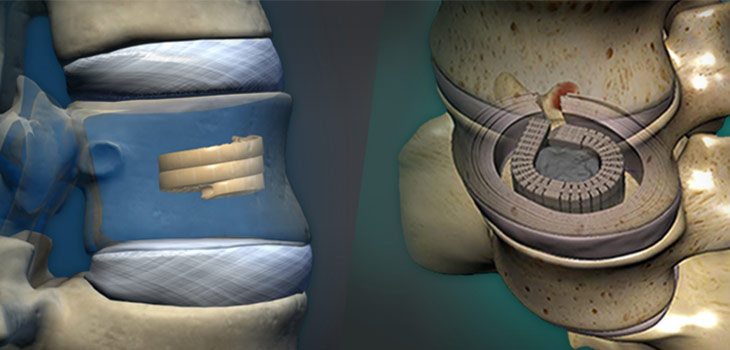
According to Exactech Chief Executive Officer David Petty, “We are pleased to broaden the use of our ExactechGPS computer guidance system with an application for revision total knee replacement. Even with the best implant systems in the hands of the best surgeons, sometimes patients’ existing implants need to be removed and replaced, due to trauma, tumors, infection or implant failure. These ‘revision’ procedures can be challenging and complex. Exactech is committed to developing innovations that can improve patient outcomes for these difficult cases, and within the last 12 months we also launched three new revision implant systems, including the Optetrak Logic® CC Comprehensive Revision Knee System.”
To develop this technology, the company partnered with orthopaedic surgeons, including Bernard Stulberg, MD, of St. Vincent Charity Medical Center in Cleveland, Ohio, who was the first to use the new ExactechGPS revision knee application last week.
“This time last year, I had the privilege of performing the first surgery with Exactech’s new Logic CC revision knee implant system and now I am honored and pleased to report a successful first surgery using the ExactechGPS revision knee application,” said Bernard Stulberg, MD. “I am impressed with the information this computer-assisted surgery system provided me during the case. The revision knee application confirmed the position and alignment of the implants, resulting in a stable and well-aligned knee. This system has great potential to improve predictability and reproducibility in revision total knee procedures.”
The ExactechGPS revision total knee application recently received 510(k) clearance in the U.S., and approval in the European Union.
The other design team members for the ExactechGPS revision knee application include Gérard Giordano, MD, of Joseph Ducuing Hospital in Toulouse, France, and James Huddleston, MD, of Stanford University Medical Center.
The Exactech revision knee system is indicated for use in skeletally mature patients undergoing surgery for total knee replacement due to osteoarthritis, osteonecrosis, rheumatoid arthritis and/or post-traumatic degenerative problems; this device is also indicated for revision of failed previous reconstruction where sufficient bone stock and soft tissue integrity are present.
About ExactechGPS
ExactechGPS® Guided Personalized Surgery is a powerful, yet compact advanced surgical technology platform that delivers efficiency and reproducibility in total joint replacement. Merging sophisticated computer guidance technology with innovative instrumentation, ExactechGPS delivers a real-time, patient-specific solution designed for improved clinical outcomes. For more information about ExactechGPS®, visit www.exactechgps.com.
About Exactech
Based in Gainesville, Fla., Exactech develops and markets orthopaedic implant devices, related surgical instruments and biologic materials and services to hospitals and physicians. The company manufactures many of its orthopaedic devices at its Gainesville facility. Exactech’s orthopaedic products are used in the restoration of bones and joints that have deteriorated as a result of injury or diseases such as arthritis. Exactech markets its products in the United States, in addition to more than 30 markets in Europe, Latin America, Asia and the Pacific. Additional information about Exactech can be found at http://www.exac.com.
*The ExactechGPS is intended for use during preoperative planning and during stereotaxic surgery to aid the surgeon in locating anatomical structures and aligning the endoprostheses with the anatomical structures.
1Hopkins AR, Hansen UN, Amis AA, Emery R. The Effects of Glenoid Component Alignment Variations on Cement Mantle Stresses in Total Shoulder Arthroplasty. J Shoulder Elbow Surgery. 2004 Nov-Dec; 13(6):668–675.
2Gregory TM, Sankey A, Augereau B, et al. Accuracy of Glenoid Component Placement in Total Shoulder Arthroplasty and Its Effect on Clinical and Radiological Outcome in a Retrospective, Longitudinal, Monocentric Open Study. PLoS ONE. 2013 Oct; 8(10):e75791.