TÜBINGEN & TUTTLINGEN, Germany–(BUSINESS WIRE)–SHS Gesellschaft für Beteiligungsmanagement mbH is investing in EIT Emerging Implant Technologies GmbH. EIT was established in Tuttlingen, Germany in 2014 and manufactures spinal implant cages using 3D printing technology. SHS is investing funds from its fourth fund generation to finance EIT’s international growth and development of its innovative products.
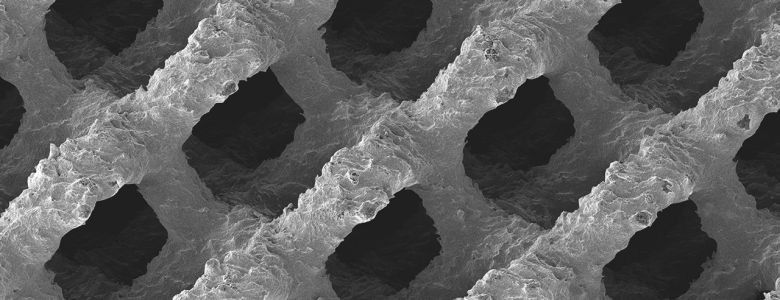
3D printing, which is also known as additive manufacturing, is used to manufacture cellular and porous implants of biocompatible titanium. These implants mimic the structure and stiffness of natural bone material more accurately than implants manufactured using traditional methods. This in turn promotes bone ingrowth following fusion operations to achieve better clinical results and reduce complication rates. In addition to this, the implants can be fitted on a patient-specific basis, which serves to increase the contact surface and reduce future risks.
“Our EIT Cellular Titanium implants provide the answer to current challenges in implant design and choice of materials as well the extreme cost pressure in medical technology. The additive manufacturing technology allows us to tackle existing problems with new solutions. Thus we can improve the benefits for patients without increasing costs, which is a clear competitive advantage”, explains Guntmar Eisen, founder and CEO of EIT. “This ensures a high level of patient satisfaction and excellent clinical results. We are looking forward to pushing ahead with our international growth, especially in the United States, and our product development with our new partner SHS.”
“EIT’s 3D-printed spine implants have already proven their superior functionality many times in practical applications, thus promoting EIT’s growth”, adds Dr. Bernhard Schirmers, Managing Partner at SHS Gesellschaft für Beteiligungsmanagement. “EIT Emerging Implant Technologies’ management team is experienced and successful in the field of spine surgery. As a medical technology investor, we look forward to supporting them on their path to increased growth.”
“With SHS, EIT is gaining a shareholder with extensive experience in this industry. Together we can enter the next stage of EIT’s growth. This includes development of the company’s innovative product portfolio as well as entering new countries”, says Guy Selbherr, Managing Director of MBG Mittelständische Beteiligungsgesellschaft Baden-Württemberg.
With a total volume of 125 million euro, the fourth SHS fund is focusing on expansion financing, changes in shareholder structures and successor situations. The Tübingen based investor is planning further acquisitions and investments in the fast-growing medical technology and life-science industries in Germany, Austria and Switzerland in the months ahead.
About EIT Emerging Implant Technologies GmbH:
EIT is the first medical device manufacturer in the orthopedic field to exclusively focus on implants that are designed and produced with additive manufacturing methods.
EIT pushes the boundaries of traditional implant manufacturing to obtain versatile, anatomically designed porous implants with increased functionality and maximal bone ingrowth capabilities for all spinal segments.
The EIT implants made of EIT Cellular Titanium® address the shortcomings of the current designs and materials, thereby ensuring optimal clinical outcomes and maximum patient satisfaction.
EIT provides a complete spinal fusion cage portfolio. Patient specific implants to help in the treatment of complex spinal disorders as well as other interesting implant concepts are in development.
EIT consists of a team of skilled and dedicated professionals with a diverse background in management, research and development and quality assurance. The team has a proven track record setting up and managing innovative spinal implant companies, and has developed an extensive network of contacts over many years including internationally renowned surgeons and opinion leaders in the field of spine surgery.
For further information, visit www.eit-spine.de/
About SHS Gesellschaft für Beteiligungsmanagement mbH
SHS Gesellschaft für Beteiligungsmanagement is based in Tübingen, Germany and invests in medical technology and life science companies with a focus on expansion financing, changes in shareholder structures and successor situations. SHS holds minority as well as majority interests. SHS was founded in 1993 and has since gained extensive experience as industry investor, which supports the growth of its portfolio companies through a network of partnerships regarding the introduction of new products, regulatory issues or entering new markets. The SHS fund’s German and international investors include about the European Investment Fund, professional pension insurers, retirement funds, funds of funds, family offices, entrepreneurs and the SHS management team. The AIFM-registered company is currently investing from its fourth generation of funds, for which investors have provided 125 million euro. Equity of up to 20 million euro is invested. Transactions can be carried out in the mid double-digit million range together with a network of co-investors. Reinhilde Spatscheck, Dr. Bernhard Schirmers, Hubertus Leonhardt and Uwe Steinbacher are the Managing Partners at SHS.
Further information: www.shs-capital.eu