News Release – March 16, 2017
HAYWARD, Calif.–(Healthcare Sales & Marketing Network)–Moximed®, Inc., developer of unicompartmental load absorber implants for active patients with painful knee osteoarthritis (OA), announced today an oversubscribed Series C round of $50MM with new investors Advent Life Sciences and Future Fund joining existing investors NEA, Morgenthaler Ventures, Gilde Healthcare, GBS Venture Partners, and Vertex Healthcare. As part of the financing, Shahzad Malik, M.D., General Partner at Advent Life Sciences, and Brigitte Smith, Managing Director at GBS Venture Partners, will join the Board of Directors.
Moximed created the category of shock absorbing implants for knee OA, with treatment durability now established to nearly nine years on the initial patients. The Moximed implants do not require bone cutting or bone removal, and, importantly, they absorb excess joint load rather than shift load to other areas of the joint. Moximed has fully enrolled its FDA pivotal clinical study of the KineSpring® System and is currently enrolling an FDA IDE clinical study of the Atlas® System.
“This financing round is timely, as we are completing the primary endpoint follow-up for our FDA pivotal study this month,” said CEO Kevin Sidow. “We expect this investment to fully fund the company through FDA approval and early US commercialization of our products. There is a massive demand for new treatment options by patients who want to maintain an active lifestyle until they are ready for a knee replacement. The tremendous patient interest and surgeon enthusiasm we are witnessing in our current Atlas IDE study is validating our effort to address this clinical need.”
“Patients between the ages of 35 and 65 years old represent the fastest growing segment of the knee OA population,” added Shahzad Malik, of Advent Life Sciences. “These patients are often considered too young for traditional joint replacement and are desperate for a treatment alternative. We are excited to support Moximed’s effort to address this opportunity.”
About Osteoarthritis
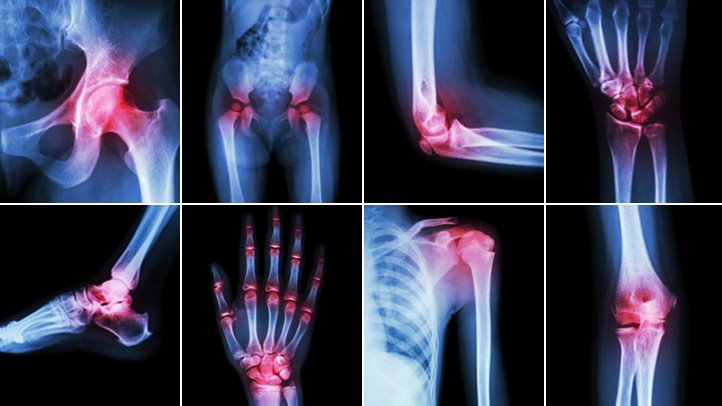
Osteoarthritis (OA), the most common form of arthritis, is a degenerative disease affecting the hands, knees, hips, feet and spine. According to a 2017 Centers for Disease Control and Prevention (CDC) report, OA affects 54 million adults (1 in 4) in the US. It is caused by changes in cartilage, the soft tissue that cushions and protects bone, leading to pain and changes in the shape of the joint. In knee OA, as the cartilage wears away, the bone ends may begin to rub against each other, causing severe pain. While often regarded as a disease of the elderly, patients between the ages of 35 – 65 years of age represent the fastest growing segment of knee OA patients.
About Moximed
Moximed, Inc. is dedicated to improving the standard of care for patients with osteoarthritis (OA). OA, the most common form of arthritis, leads to a breakdown of the joint’s cartilage and often results in joint pain and loss of motion. Moximed is supported by world-leading venture investors including NEA, Morgenthaler Ventures, Gilde Healthcare, GBS Venture Partners, Vertex Healthcare, Advent Life Sciences, and Future Fund. More information can be found at www.moximed.com.
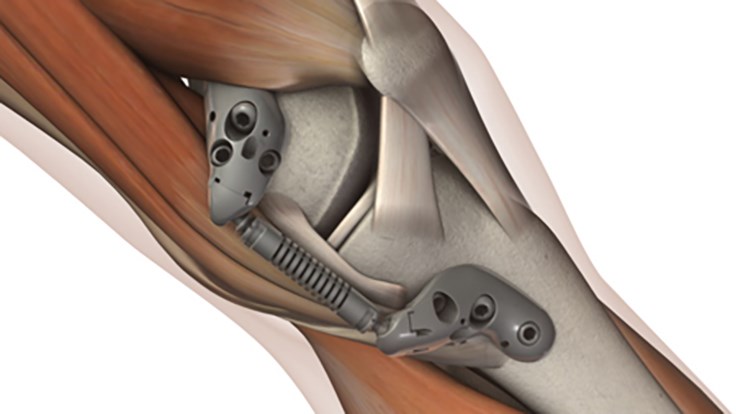
About the Atlas System
The Atlas System is designed as an implantable joint unloader for patients with unicompartmental knee osteoarthritis. Placed subcutaneously alongside the knee joint, the Atlas System incorporates advanced biomaterials designed to provide a clinically beneficial 30 lbs. of unloading. Importantly, the Atlas System absorbs excess joint load rather than shift load to otherwise healthy areas of the joint. This is important because load shifting has been shown in clinical studies to accelerate degeneration of otherwise healthy joint tissues. The Atlas System features a streamlined surgical technique that uses the patient’s own anatomy and allows the surgeon to visually confirm device function during the procedure. Finally, treatment with the Atlas System does not require any bone cutting or bone removal, and patients maintain all future treatment options should their osteoarthritis become more severe.
The Atlas System is CE Marked in Europe and is currently only being made available to select centers. The Atlas System is an investigational device in the United States, where it is limited by US law to investigational use.
About Advent Life Sciences
Advent Life Sciences founds and invests in early- and mid-stage life sciences companies that have a first- or best-in-class approach to unmet medical needs. The investing team consists of experienced professionals, each with extensive scientific, medical and operational experience, a long-standing record of entrepreneurial and investment success in the US and Europe, and is particularly focused on supporting entrepreneurs and founders to take innovative new medical entities from concept to approval. The Firm invests in a range of sectors within life sciences, principally drug discovery, enabling technologies and med tech, always with an emphasis on innovative, paradigm-changing approaches. Advent Life Sciences has a presence in the UK, US and France. For more information, please visit www.AdventLS.com
About Future Fund
The Future Fund is Australia’s sovereign wealth fund and invests for the benefit of future generations of Australians. The Future Fund was established in 2006 and has taken on management of additional public asset funds, including the Disability Care Australia Fund, the Medical Research Future Fund, and two Nation-building Funds. The Fund’s role is to generate high, risk-adjusted returns over the long-term. The Fund operates independently from Government and tailors the management of each fund to its unique investment mandate.
Source: Moximed
Issuer of this News Release is solely responsible for its content.
Please address inquiries directly to the issuing company.