DUBLIN–(BUSINESS WIRE)–3D4Medical, the world’s leading developer of 3D medical technology, today unveiled the first in its new suite of clinical solutions with the Complete Ortho app for iPhone and iPad, available exclusively on the App Store.
“Complete Consultation” is the new range of clinical solutions from 3D4Medical that will cover areas such as orthopedics, cardiology, internal medicine and trauma. Aiming to transform the relationship between the healthcare professional and patient, the first product in the range, Complete Ortho, allows the healthcare professional to consult with the patient in a whole new way, both educating and empowering them at every step of their orthopedic healthcare journey in magnificent 3D and all across a fully HIPAA-compliant platform.
First introduced in 2013 in one of the largest and most well-known hospital chains in the US, Complete Ortho was particularly well-received. 3D4Medical is now delighted to bring Complete Ortho to individual orthopedic surgeons and clinics around the world and for free to the general public so everyone can directly benefit.
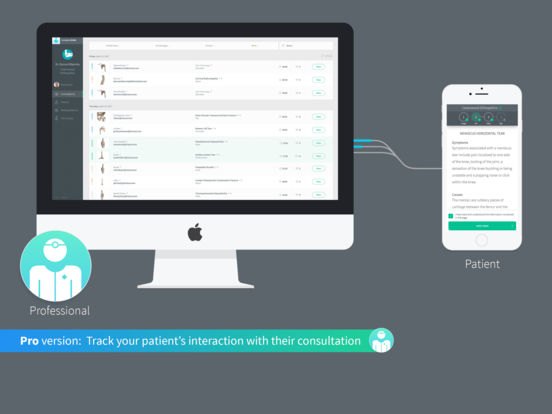
Complete Ortho streamlines and enhances the entire healthcare professional/patient consultation process, allowing the healthcare professional to take their patient through the entire orthopedic concern with the aid of ground-breaking 3D technology and animations: from the anatomy of the troubling region; the possible pathologies related to the structure in question; the potentially beneficial or appropriate procedures; and any additional information that might be relevant. The patient is better engaged and empowered like never before as the step-by-step process allows for a far greater level of understanding than those achieved by traditional methods. If the patient so wishes, the healthcare professional can email a summary of their information, including the 3D animations, to them in the form of a Digital Consultation for their review on any device, in their own time and space, and perhaps with their loved ones for added support, removing the stress of trying to remember everything that the healthcare professional said, and increasing the accuracy of the information that is informing the patient’s decision. This improvement in the level of shared decision-making, education and empowerment will no doubt lead to a decrease in unnecessary physician return visits, an increase in patient-satisfaction and better medical outcomes across the board.
CEO and founder of 3D4Medical, John Moore, said, “We have had Complete Ortho ready for the hospital setting for a long time now so it is great to finally get it into the hands of both healthcare professionals and the general public. We look forward to the positive impact it will make across the world.”
Alan Getgood of the University of Western Ontario and Canada’s Fowler Kennedy Sport Medicine Clinic noted, “Complete Ortho will provide an excellent resource for physicians to provide a more personalized information portfolio for their patients’ diagnosis and treatment plan. For the patient, this will ensure a better understanding of their prognosis and care pathway that should lead to improved levels of satisfaction.”
Maurice Neligan, Director of Orthopaedic Surgery at Ireland’s Beacon Hospital and Associate Clinical Professor at University College Dublin School of Medicine, stated, “Complete Ortho is a significant advancement in engaging patients. It dispenses with the need for plastic models and scribbled diagrams, replacing them with top-quality illustrations, animations, and information that are personalized to the patient’s pathology and treatment. It is well-known that better-engaged patients have better outcomes and the information generated from patient engagement with Complete Ortho allows a more robust consent process for treatment, lowering the risk of malpractice litigation and the process can be seamlessly incorporated into existing practice models with little or no increase in consultation time.”
International expert in hip and knee joint replacement and Director of the Australian Orthopedic Association National Joint Replacement Registry, Steve Graves, added, “3D technology is proven to be the most effective approach to inform patients about their clinical condition. Complete Ortho is a comprehensive high-quality product that will greatly assist surgeons to ensure that their patients are fully-informed about their condition and proposed management.”
3D4Medical is proud to be an Apple Mobility Partner, helping to deliver best-in-class iOS solutions like Complete Ortho to healthcare professionals and patients around the world. Complete Ortho is exclusively available for iPad Mini2, iPad Mini3, iPad Mini4, iPad Air1, iPad Air2, iPad Pro, iPhone SE, iPhone 5S, iPhone 6, 6+, 6S, 6S+ and 7.
About 3D4Medical
3D4Medical is transforming medical learning and practice across the world and is leading the way in the production of ground-breaking 3D medical technology applications. This technology disrupts traditional methods of education by providing revolutionary applications that allow the educator, student, medical professional and patient to explore and experience medical education like never before, putting high-quality accessible 3D medical information at their fingertips. With the receipt of a prestigious Apple Design Award in 2016, over 12 million downloads worldwide and the #1 top-download positions in the App Store in 148 countries, 3D4Medical has enjoyed great success to date as it continues in its pursuit to improve the lives of patients, students, medical professionals and educators around the world. 3D4Medical is headquartered in Dublin and has over 100 employees.