SAN JOSE, Calif., June 12, 2017 /PRNewswire/ — SI-BONE, Inc., an innovative medical device company that pioneered the use of the iFuse Implant System® (“iFuse”), a triangular shaped minimally invasive surgical (MIS) device indicated for fusion for certain disorders of the sacroiliac (SI) joint, announced that Blue Cross and Blue Shield of Vermont (BCBSVT), an independent, local, not-for-profit Vermont company, has established a written positive coverage policy for minimally invasive surgical (MIS) SI joint fusion. The policy, available at the link below, will provide coverage for more than 200,000 BCBSVT health plan members and will become effective on July 1, 2017. BCBSVT becomes the fourth BCBS insurer to cover MIS SI joint fusion joining the BCBS insurers HCSC (that includes BCBS of Illinois, Montana, New Mexico, Oklahoma and Texas), BCBS of Michigan and BCBS of Nebraska bringing the total number of people with BCBS insurance who have access to the procedure to over 18 million in eight states.
Craig Bartlett, MD of the University of Vermont Medical Group in Burlington, VT commented: “This is great news and I am delighted for our SI joint patients with Blue Cross Blue Shield of Vermont coverage. I regularly see patients in my practice with chronic sacroiliac joint pain who could benefit from minimally invasive sacroiliac joint fusion with iFuse but I have been unable to perform the procedure due to coverage limitations. Now, with this positive coverage decision, I will be able to provide iFuse as a treatment option to Blue Cross Blue Shield of Vermont patients who are properly diagnosed surgical candidates and meet the treatment criteria.”
“We are very pleased to learn that Blue Cross Blue Shield of Vermont has established a positive coverage policy based on the published peer-reviewed clinical evidence for patients with SI joint related low back or buttock pain who essentially meet the NASS coverage criteria for MIS SI joint fusion,” said Michael Mydra, Vice President, Health Outcomes & Reimbursement at SI-BONE.
About SI joint dysfunction
The SI joint has been attributed as a source of pain in 15-30 percent of patients with chronic low back pain1-4, and in up to 43 percent of patients with new onset or persistent low back pain after lumbar fusion.5 Like all other major joints, the SI joint can be injured or degenerate, which can cause debilitating pain in the lower back, buttocks and legs. Simple movements such as standing up, sitting down, stepping up or down, bending and lifting, walking, or even sleeping or sitting on the affected side can provoke a symptomatic SI joint.
SI joint dysfunction is often misdiagnosed and the resulting pain can be misattributed to other causes. Not all healthcare providers evaluate the SI joint and many patients do not know to ask about it. While not commonly diagnosed, SI joint disorders can be identified when a patient points to their source of pain directly over the posterior superior iliac spine (PSIS) known as the Fortin Finger Sign, combined with a number of positive provocative maneuvers to stress the SI joint and elicit the pain, followed by image-guided diagnostic injections.
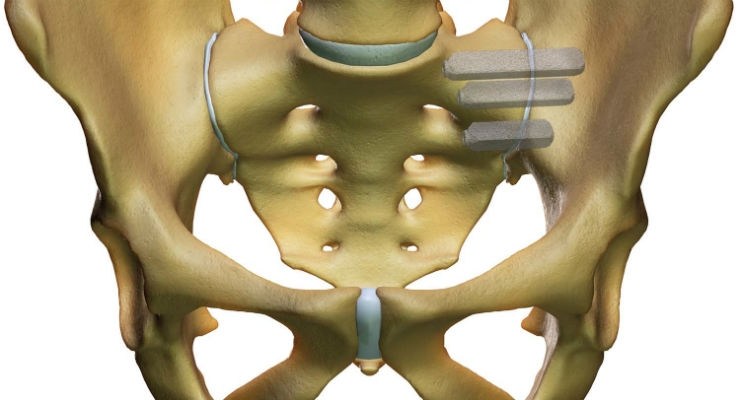
The other major joints in the human body, such as knees, hips, ankles and shoulders, have specialized device-based surgical solutions. The SI joint is the largest and the last of eight major joints in the human body to have a proven surgical solution. The iFuse ImplantTMwas designed specifically to withstand the extreme forces resulting from load-bearing and the unique rotational and translational motion of the SI joint referred to as nutation, and is supported by more than 50 peer reviewed publications including two level 1 randomized controlled trials.
About SI-BONE, Inc.
SI-BONE, Inc. (San Jose, California) is a leading medical device company that has developed the iFuse Implant System, a proprietary minimally invasive surgical implant system to fuse the sacroiliac joint to treat common disorders of the joint that can cause lower back pain. Patients with sacroiliac joint dysfunction experience pain that can be debilitating. SI-BONE believes that the sacroiliac joint is the last of the eight major joints in the human body to have a proven surgical treatment and that the iFuse Implant is the only device for treatment of SI joint dysfunction supported by significant published clinical evidence, including level 1 trials, showing safety and durable effectiveness, including providing lasting pain relief.
The iFuse Implant System is intended for sacroiliac fusion for conditions including sacroiliac joint dysfunction that is a direct result of sacroiliac joint disruption and degenerative sacroiliitis. This includes conditions whose symptoms began during pregnancy or in the peripartum period and have persisted postpartum for more than 6 months. There are potential risks associated with the iFuse Implant System. It may not be appropriate for all patients and all patients may not benefit. For information about the risks, visit: www.si-bone.com/risks
SI-BONE and iFuse Implant System are registered trademarks of SI-BONE, Inc. ©2017 SI-BONE, Inc. All Rights Reserved. 9929.061217
- Bernard TN, Kirkaldy-Willis WH. Recognizing specific characteristics of nonspecific low back pain. Clin Orthop Relat Res. 1987;217:266–80.
- Schwarzer AC, Aprill CN, Bogduk N. The Sacroiliac Joint in Chronic Low Back Pain. Spine. 1995;20:31–7.
- Maigne JY, Aivaliklis A, Pfefer F. Results of Sacroiliac Joint Double Block and Value of Sacroiliac Pain Provocation Tests in 54 Patients with Low Back Pain. Spine. 1996;21:1889–92.
- Sembrano JN, Polly DW Jr. How Often is Low Back Pain Not Coming From The Back? Spine. 2009;34:E27–32.
- DePalma M, Ketchum JM, Saullo TR. Etiology of Chronic Low Back Pain Patients Having Undergone Lumbar Fusion. Pain Med. 2011;12:732–9.
SOURCE SI-BONE, Inc.