June 27, 2017
INDIANAPOLIS–(BUSINESS WIRE)–OrthoIndy and Active Implants, a company that develops orthopedic implant solutions, today announced that the first meniscus replacement procedures have been performed in Indiana. Dr. Jack Farr, orthopedic surgeon at OrthoIndy, was the first to perform the procedure on Greenwood resident Jason Scott. OrthoIndy is the only center in the state – and just one of 10 sites nationwide – participating in the VENUS (Verification of the Effectiveness of the NUsurface® System) clinical trial, which is enrolling patients with persistent knee pain after loss of meniscus cartilage to assess the safety and effectiveness of the investigational NUsurface Meniscus Implant compared to non-surgical standard of care.
The meniscus is a tissue pad between the thigh and shin bones. Once it is damaged, the meniscus has a very limited ability to heal. Over 1 million partial meniscectomies (to remove the torn portion of the meniscus) are performed in the U.S. every year, more than the total number of hip and knee replacement surgeries combined. However, many patients still experience persistent knee pain following meniscus partial excision surgery.
Scott suffered a knee injury resulting in a meniscus tear while playing high school basketball 18 years ago. This injury led the 36-year-old to undergo two partial meniscectomy surgeries on his left knee, which allowed him initially to get back to playing basketball and softball. However, the knee pain flared up over the past few years to where Scott began to experience consistent knee pain during any activity, including participating in sports, playing with his kids and simply sitting in the car for too long.
“We don’t have many surgical options for patients like Jason,” Dr. Farr said. “He is too young for knee replacement surgery and was not interested in the time it takes to recover from tibial realignment or meniscal transplant surgery. We hope NUsurface not only alleviates his pain and gets him back to his normal activities, but possibly helps him delay or avoid knee replacement surgery.”
Dr. Farr implanted the device in May 2016 through a small incision in Scott’s knee. Following a full six-week physical therapy regimen,Scott was able to gain full range of motion as well as resume daily activities. It has been one year since he received the implant and Scott’s knee continues to be stable. He has been able to play basketball again, go to concerts and sporting events, and take family vacations without worrying about knee pain.
“I feel fortunate that at the young age of 36, I’m able to receive a meniscus implant instead of living with persistent pain that could lead to an unwanted knee replacement later in life,” Scott said. “As a father of two young girls, I need to be as mobile and active as my kids are – the NUsurface implant has helped to alleviate my knee pain so I can play sports with my kids, travel with my family and get back to my athletic lifestyle.”
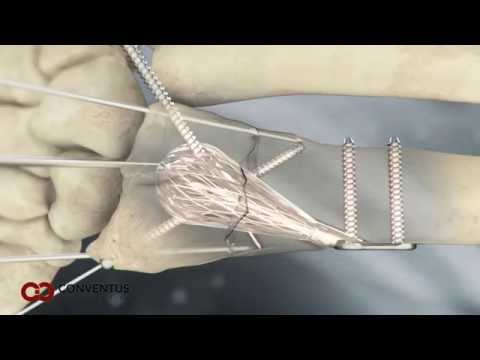
The NUsurface Meniscus Implant is inserted into the knee joint through a small incision and patients typically can go home on the same day of the operation. After surgery, they undergo a six-week rehabilitation program. NUsurface has been used in Europe since 2008 and Israel since 2011.
About the Clinical Trial
As part of the process to gain regulatory approval in the U.S., the VENUS (Verification of the Effectiveness of the NUsurface® System) study will enroll approximately 130 patients at orthopedic centers in the U.S., Europe and Israel. Sites in the U.S. include Indiana (Indianapolis), Massachusetts (Boston), New York (Albany, Rochester and New York), North Carolina (Durham), Ohio (Columbus), Tennessee (Memphis) and Virginia (Richmond). Participants who meet study requirements and agree to enter the trial are randomized to receive either the NUsurface device or non-surgical treatment, which is the current standard of care for patients with persistent knee pain following meniscus surgery. To be eligible for the study, participants must be between the ages of 30 and 75 and have pain after medial meniscus surgery that was performed at least six months ago. To learn more about the VENUS study, please call (844) 680-8951 or visit www.meniscus-trial.com.
About the NUsurface® Meniscus Implant
In the U.S., the NUsurface® Meniscus Implant, from Active Implants LLC, is an investigational treatment for patients with persistent knee pain following medial meniscus surgery. The NUsurface Meniscus Implant is made from medical grade plastic and, as a result of its unique materials, composite structure and design, does not require fixation to bone or soft tissue. The NUsurface device mimics the function of the natural meniscus and redistributes loads transmitted across the knee joint. It is inserted into the knee joint through a small incision, and patients typically can go home soon after the operation. After surgery, patients undergo a six-week rehabilitation program. The NUsurface device has been used clinically in Europe since 2008 and Israel since 2011.
About OrthoIndy and the OrthoIndy Hospital
Founded over 50 years ago, OrthoIndy is one of the most highly respected orthopedic practices in the Midwest. With over 70 physicians providing care to central Indiana residents from more than 10 convenient locations, OrthoIndy provides leading-edge bone, joint, spine and muscle care. OrthoIndy physicians also provide care to the Indiana Pacers, Indiana Fever, Indianapolis Indians and Dance Kaleidoscope, as well as local colleges and high schools.
Owned and operated by OrthoIndy physicians, OrthoIndy Hospital is central Indiana’s first specialty hospital with a direct focus on bone, joint, spine and muscle care and the most technologically advanced facility available for orthopedics in the Midwest. As a physician-owned hospital model, OrthoIndy Hospital’s top priorities are patient safety, satisfaction and outcomes.
Quality patient care serves as the foundation for the physician-owned and operated hospital, which is evident in the ratings received from Press Ganey Associates, Inc. From 2013 to 2016 OrthoIndy Hospital was named a Guardian of Excellence Award winner for consistently achieving the 95th percentile of performance in patient satisfaction. From 2009 to 2012, OrthoIndy Hospital was named the Summit Award Winner for sustaining the highest level of customer satisfaction for three or more consecutive years.
For more information on OrthoIndy, please call (317) 802-2000 or visit http://orthoindy.com/.
About Active Implants
Active Implants, LLC develops orthopedic implant solutions that complement the natural biomechanics of the musculoskeletal system, allowing patients to maintain or return to an active lifestyle. Active Implants is privately held with headquarters in Memphis, Tennessee. European offices are in The Netherlands, with R&D facilities in Israel. For more information, visit www.activeimplants.com.
CAUTION Investigational device. Limited by United States law to investigational use.
Contacts
Merryman Communications
Joni Ramirez, 323-532-0746
joni@merrymancommunications.com






 About Bone Therapeutics
About Bone Therapeutics