CAMBRIDGE, Mass.–(BUSINESS WIRE)–InVivo Therapeutics Holdings Corp. (NVIV) today provided a general business update and reported financial results for the quarter ended September 30, 2017.
Mark Perrin, InVivo’s Chief Executive Officer and Chairman, said, “In the third quarter, we focused our resources solely on reopening enrollment into the clinical study of the Neuro-Spinal Scaffold™ in complete thoracic spinal cord injury (SCI). As part of this focus, we reduced our workforce by 39%, which is expected to result in 2018 operating expense savings of approximately $7.3 million.”
Mr. Perrin continued, “Enrollment into the INSPIRE study is currently on hold as we engage with the United States Food & Drug Administration (FDA) to determine the most appropriate clinical path forward. We continue to engage in discussions with the FDA regarding our clinical program and will provide appropriate updates as we obtain further clarity on the issue. We remain wholly committed to our mission of redefining the life of the spinal cord injury patient and progressing the Neuro-Spinal Scaffold in the clinic toward a Humanitarian Device Exemption (HDE) submission.”
INSPIRE Update
Of the 19 patients implanted in The INSPIRE Study to date, 16 patients are in follow-up, 14 of whom have reached the six-month primary endpoint visit. Six of these 14 (42.9%) have improved from complete AIS A SCI to incomplete SCI (one patient to AIS C and five patients to AIS B) at the six-month primary endpoint visit and eight had not converted at that visit. Two of the six patients who converted and were assessed to have AIS B SCI at the six-month primary endpoint were later assessed to have improved to AIS C SCI at the 12 and 24-month visits, respectively. Two of the patients in follow-up have not yet reached the six-month primary endpoint visit although one of these patients was assessed to be AIS C at one month and two months and AIS B at three months. Three patients in the INSPIRE study have died, with the cause of death in each case determined to be unrelated to the Neuro-Spinal Scaffold or implant procedure by the respective site Principal Investigator.
Financial Results
For the three-month period ended September 30, 2017, the Company reported a net loss of approximately $9.4 million, or $0.28 per diluted share, compared to a net loss of $6.2 million, or $0.19 per diluted share, for the three-month period ended September 30, 2016. The results for the three-month period ended September 30, 2017 were unfavorably impacted by increases in general and administrative operating expenses of $804,000 and by a derivative loss of $3.1 million, due primarily to the impact of the August 2017 warrant exchange, partially offset by decreases in research and development operating expenses of $366,000. Excluding the impact of the derivative warrant liability, adjusted net loss for the three-month period ended September 30, 2017 was $6.3 million, or $0.19 per diluted share, compared to adjusted net loss of $5.9 million, or $0.18 per diluted share, for the three-month period ended September 30, 2016.
The Company ended the quarter with $17.2 million of cash, cash equivalents, and marketable securities.
For the nine-month period ended September 30, 2017, the Company reported a net loss of approximately $22.1 million, or $0.68 per diluted share, compared to a net loss of $18.0 million or $0.59 per diluted share, for the nine-month period ended September 30, 2016. The results for the nine-month period ended September 30, 2017 were unfavorably impacted by increases in research and development operating expenses of $863,000, $1.8 million in general and administrative operating expenses and by a non-cash loss on the derivative warrant liability of $2.3 million, due to the impact of the August 2017 warrant exchange and the change in the fair market value of the warrant liability. Excluding the impact of the derivative warrant liability, adjusted net loss for the nine-month period ended September 30, 2017 was $19.8 million, or $0.61 per diluted share, compared to adjusted net loss of $17.2 million, or $0.56 per diluted share, for the nine-month period ended September 30, 2016.
Adjusted net loss and adjusted net loss per share are non-GAAP financial measures that exclude the impact of the derivative warrant liability. A reconciliation of these measures to the comparable GAAP measure is included with the tables contained in this release. The Company believes a presentation of these non-GAAP measures provides useful information to investors to better understand the Company’s operations, on a period-to-period comparable basis, with financial amounts both including and excluding the identified items.
About The INSPIRE Study
The INSPIRE Study: InVivo Study of Probable Benefit of the Neuro-Spinal Scaffold™ for Safety and Neurologic Recovery in Subjects with Complete Thoracic AIS A Spinal Cord Injury, is designed to demonstrate the safety and probable benefit of the Neuro-Spinal Scaffold™ for the treatment of complete T2-T12/L1 spinal cord injury in support of a Humanitarian Device Exemption (HDE) application for approval.
About the Neuro-Spinal Scaffold™ Implant
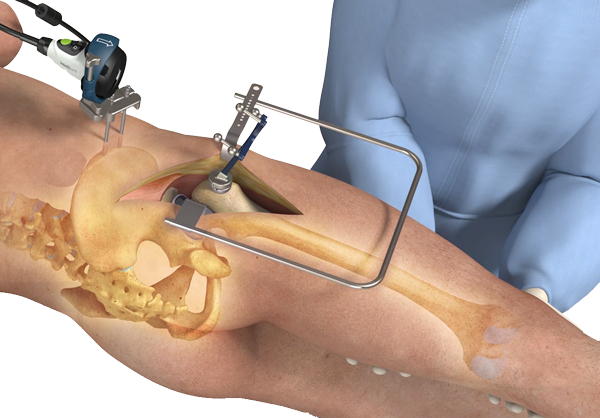
Following acute spinal cord injury, surgical implantation of the biodegradable Neuro-Spinal Scaffold™ within the decompressed and debrided injury epicenter is intended to support appositional healing, thereby reducing post-traumatic cavity formation, sparing white matter, and allowing neural repair within and around the healed wound epicenter. The Neuro-Spinal Scaffold™, an investigational device, has received a Humanitarian Use Device (HUD) designation and currently is being evaluated in The INSPIRE Study for the treatment of patients with acute, complete (AIS A), thoracic traumatic spinal cord injury.
About InVivo Therapeutics
InVivo Therapeutics Holdings Corp. is a research and clinical-stage biomaterials and biotechnology company with a focus on treatment of spinal cord injuries. The company was founded in 2005 with proprietary technology co-invented by Robert Langer, Sc.D., Professor at Massachusetts Institute of Technology, and Joseph P. Vacanti, M.D., who then was at Boston Children’s Hospital and who now is affiliated with Massachusetts General Hospital. In 2011, the company earned the David S. Apple Award from the American Spinal Injury Association for its outstanding contribution to spinal cord injury medicine. In 2015, the company’s investigational Neuro-Spinal Scaffold™received the 2015 Becker’s Healthcare Spine Device Award. The publicly-traded company is headquartered in Cambridge, MA. For more details, visit www.invivotherapeutics.com.
Safe Harbor Statement
Any statements contained in this press release that do not describe historical facts may constitute forward-looking statements within the meaning of the federal securities laws. These statements can be identified by words such as “believe,” “anticipate,” “intend,” “estimate,” “will,” “may,” “should,” “expect,” “designed to,” “potentially,” and similar expressions, and include statements regarding the status of the clinical program, the timing for re-opening enrollment in the INSPIRE Study and the submission of an HDE application to the FDA, and the impact of the restructuring and the reduction in force on the Company’s balance sheet, financial position and cash burn. Any forward-looking statements contained herein are based on current expectations, and are subject to a number of risks and uncertainties. Factors that could cause actual future results to differ materially from current expectations include, but are not limited to, risks and uncertainties relating to the company’s ability to successfully re-open clinical sites for enrollment and to enroll additional patients; the timing of the Institutional Review Board process; the expected benefits and efficacy of the company’s products and technology in connection with the treatment of spinal cord injuries; the availability of substantial additional funding for the company to continue its operations and to conduct research and development, clinical studies and future product commercialization; and other risks associated with the company’s business, research, product development, regulatory approval, marketing and distribution plans and strategies identified and described in more detail in the company’s Quarterly Report of the three months ended September 30, 2017, and its other filings with the SEC, including the company’s Form 10-Qs and current reports on Form 8-K. The company does not undertake to update these forward-looking statements.
|
| InVivo Therapeutics Holdings Corp. |
| Consolidated Balance Sheets |
| Unaudited |
|
|
|
|
|
|
|
|
|
|
As of |
|
|
|
September 30,
2017
|
|
|
December 31,
2016
|
| ASSETS: |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Current assets: |
|
|
|
|
|
|
| Cash and cash equivalents |
|
|
16,434 |
|
|
|
21,464 |
|
| Restricted cash |
|
|
361 |
|
|
|
361 |
|
| Marketable securities |
|
|
734 |
|
|
|
11,577 |
|
| Prepaid expenses and other current assets |
|
|
475 |
|
|
|
451 |
|
| Total current assets |
|
|
18,004 |
|
|
|
33,853 |
|
| Property, equipment and leasehold improvements, net |
|
|
190 |
|
|
|
510 |
|
| Other assets |
|
|
402 |
|
|
|
421 |
|
| Total assets |
|
|
18,596 |
|
|
|
34,784 |
|
|
|
|
|
|
|
|
| LIABILITIES AND STOCKHOLDERS’ EQUITY: |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Current liabilities: |
|
|
|
|
|
|
| Accounts payable |
|
|
1,270 |
|
|
|
1,011 |
|
| Loan payable, current portion |
|
|
444 |
|
|
|
423 |
|
| Derivative warrant liability |
|
|
41 |
|
|
|
1,314 |
|
| Deferred rent, current portion |
|
|
30
|
|
|
|
141 |
|
| Accrued expenses |
|
|
1,784 |
|
|
|
1,959 |
|
| Total current liabilities |
|
|
3,569 |
|
|
|
4,848 |
|
| Loan payable, net of current portion |
|
|
516 |
|
|
|
852 |
|
| Deferred rent, net of current portion |
|
|
208 |
|
|
|
135 |
|
| Other liabilities |
|
|
52 |
|
|
|
— |
|
| Total liabilities |
|
|
4,345 |
|
|
|
5,835 |
|
|
|
|
|
|
|
|
| Stockholders’ equity: |
|
|
|
|
|
|
|
Common stock, $0.00001 par value, authorized 100,000,000 shares; 34,234,580 shares
issued and outstanding at September 30, 2017; 32,044,087 shares issued and outstanding at
December 31, 2016
|
|
|
1
|
|
|
|
1
|
|
| Additional paid-in capital |
|
|
193,493 |
|
|
|
185,955 |
|
| Accumulated deficit |
|
|
(179,243 |
) |
|
|
(157,007 |
) |
| Total stockholders’ equity |
|
|
14,251 |
|
|
|
28,949 |
|
| Total liabilities and stockholders’ equity |
|
|
18,596 |
|
|
|
34,784 |
|
|
|
|
|
|
|
|
|
InVivo Therapeutics Holdings Corp.
Consolidated Statements of Operations and Comprehensive Loss
(Unaudited)
|
|
|
|
|
Three Months Ended
September 30,
|
|
|
Nine Months Ended
September 30,
|
|
|
|
2017 |
|
|
|
2016 |
|
|
|
2017 |
|
|
|
2016 |
|
| Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
| Research and development |
|
|
2,928 |
|
|
|
3,294 |
|
|
|
9,522 |
|
|
|
8,659 |
|
| General and administrative |
|
|
3,388 |
|
|
|
2,584 |
|
|
|
10,389 |
|
|
|
8,573 |
|
| Total operating expenses |
|
|
6,316 |
|
|
|
5,878 |
|
|
|
19,911 |
|
|
|
17,232 |
|
| Operating loss |
|
|
(6,316 |
) |
|
|
(5,878 |
) |
|
|
(19,911 |
) |
|
|
(17,232 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
| Interest income |
|
|
43 |
|
|
|
50 |
|
|
|
152 |
|
|
|
133 |
|
| Interest expense |
|
|
(18 |
) |
|
|
(32 |
) |
|
|
(58 |
) |
|
|
(117 |
) |
| Derivatives gain (loss) |
|
|
(3,059 |
) |
|
|
(336 |
) |
|
|
(2,264 |
) |
|
|
(788 |
) |
| Other income (expense), net |
|
|
(3,034 |
) |
|
|
(318 |
) |
|
|
(2,170 |
) |
|
|
(772 |
) |
| Net loss |
|
|
(9,350 |
) |
|
|
(6,196 |
) |
|
|
(22,081 |
) |
|
|
(18,004 |
) |
| Net loss per share, basic and diluted |
|
|
(0.28 |
) |
|
|
(0.19 |
) |
|
|
(0.68 |
) |
|
|
(0.59 |
) |
| Weighted average number of |
|
|
|
|
|
|
|
|
|
|
|
|
| common shares outstanding, basic and diluted |
|
|
33,445,002 |
|
|
|
31,968,357 |
|
|
|
32,516,190 |
|
|
|
30,687,263 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Reconciliation of GAAP to non-GAAP measures |
| InVivo Therapeutics Holdings Corp. |
| (In thousands, except share and per share data) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended |
|
|
Nine Months Ended |
|
|
|
September 30, |
|
|
September 30, |
|
|
|
2017 |
|
|
2016 |
|
|
2017 |
|
|
2016 |
| Reported GAAP net income (loss) |
|
|
(9,350 |
) |
|
|
(6,196 |
) |
|
|
(22,081 |
) |
|
|
(18,004 |
) |
| Add Back: Derivative (Gain)/ Loss |
|
|
3,059 |
|
|
|
336 |
|
|
|
2,264 |
|
|
|
788 |
|
| Adjusted Net Loss |
|
|
(6,291 |
) |
|
|
(5,860 |
) |
|
|
(19,817 |
) |
|
|
(17,216 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Reported GAAP net loss per diluted share |
|
|
(0.28 |
) |
|
|
(0.19 |
) |
|
|
(0.68 |
) |
|
|
(0.59 |
) |
| Derivative loss per diluted share |
|
|
0.09 |
|
|
|
0.01 |
|
|
|
0.07 |
|
|
|
0.03 |
|
| Adjusted net loss per diluted share |
|
|
(0.19 |
) |
|
|
(0.18 |
) |
|
|
(0.61 |
) |
|
|
(0.56 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|