May 17, 2017
TOULOUSE, France–(BUSINESS WIRE)–Regulatory News:
VEXIM (Paris:ALVXM) (FR0011072602 – ALVXM), a medical device company specializing in the minimally invasive treatment of vertebral fractures, today announces its fifth-year anniversary on the Alternext market.
“After five years of quotation on the Alternext market, VEXIM has made tremendous progress on all fronts. VEXIM has successfully implemented its direct go to market strategy in Europe reaching 10% market share in 2016 and reached profitability on the second half of 2016. VEXIM also attracted and recruited more than 30 talented and experienced employees supporting the growth of VEXIM’s business and serving our customers every day. We also generated value for our shareholders by tripling our market capitalization in 5 years. With the US market, EU market, international expansion and our product portfolio extension projects, VEXIM has a great potential of growth for the next coming years. We would like to thank all VEXIM’s shareholders from the first days and the ones who have joined recently for their trust and support“, said Vincent Gardès, CEO of VEXIM.
EUROPE, A GREAT SUCCESS IN 5 YEARS
In 2012, we launched our Go-to-market strategy in Europe that was to address 90% of the market through a direct sales force organization. After 5 years, VEXIM results are impressive with a compounded average growth rate of our sales of 74% since 2011. We now have reached a 10% market share position1 as of end of 2016 and a leading market position in France2, VEXIM’s domestic market. We aim to continue to gain market share in the coming years in Europe by focusing mainly in Germany and all other countries.
EXPANDING OUR PRODUCT PORTFOLIO
The SpineJack® remains VEXIM’s main product representing close to 90% of our sales but in the past 5 years we also launched new products to extend our portfolio. The Interface™ launched in 2014, a cement with 50% of Hydroxyapatite to be used in younger patients. The Masterflow™ launched in 2015, is a sophisticated solution providing a controlled high viscosity cement injection, at a safe distance3from the radiation field for multi-level procedures. And more recently the Masterflow™ PLUS launched in March 2017 in Germany that allows a Controlled Cement Augmentation Procedure first through vertebral body height elevation, and second by stabilizing the fracture using Cohesion® Bone Cement injection. We have also other projects in the pipeline to extend our portfolio into high energy vertebral fractures and tumor. This promising portfolio will strenghthen our Spine trauma positioning and expertize.
BUILDING CLINICAL EVIDENCE
We have successfully conducted 7 international clinical studies and 4 biomechanical studies to support the efficacy and safety of our SpineJack® product. These studies have led to 14 publications in renowned medical journals4. VEXIM is continuously looking and investing to build more clinical evidence. VEXIM’s US FDA 510K clinical study, the clinical and health economic study comparing the SpineJack® vs conservative treatments and the future clinical study in Germany where the SpineJack® will be more evidence based towards a wider range of indications to follow, are demonstrating VEXIM’s commitment to build clinical evidence and demonstrate benefits of its technology.
HIGH PERFORMING ORGANIZATION
VEXIM’s culture of execution, passion and customer focus are key strengths that supported the development of the company on the past 5 years. Vexim more than doubled its number of employees in 5 years reaching 66 employees across all entities with diverse and experienced talents across the company. VEXIM will continue to recruit talents across the globe to sustain its objectives and projects.
STRATEGIC INTERNATIONAL EXPANSION
There has been a growing interest in the SpineJack® across the world. The SpineJack® is today available in more than 15 countries across the world from Central & Latin America to Eastern Europe, Middle East, South Africa and South East Asia. We will continue to expand our footprint by opening new markets such as Brazil, Australia, South Korea, China.
OPERATIONAL AND FINANCIAL EXCELLENCE
VEXIM successfully achieved to turn profitable on a full semester in the second half of 2016 and generated positive cash flows. VEXIM also increased its Gross Margin on sales from 59% to 72% in average by optimizing its production costs and inventory processes. VEXIM aims at reaching profitability on a full year basis in 2017 and future cash flows should allow VEXIM to self-finance, in line with its ambitions.
Click here to consult VEXIM’s 2016 results presentation
Financial reporting schedule:
2nd quarter sales: July 11th, 20175
About VEXIM, the innovative back microsurgery specialist
Based in Balma, near Toulouse (France), VEXIM is a medical device company created in February 2006. The Company has specialized in the creation and marketing of minimally invasive solutions for treating traumatic spinal pathologies. Benefitting from the financial support of it longstanding shareholder, Truffle Capital6, and from OSEO public subsidies, VEXIM has designed and developed the SpineJack®, a unique implant capable of repairing a fractured vertebra and restoring the balance of the spinal column. The company also developed the MasterflowTM, an innovative solution for mixing and injecting orthopedic cement that enhances the accuracy of the injection and optimizes the overall surgical procedure. The company counts 66 employees, including its own sales teams in Europe and a network of international distributors. VEXIM has been listed on NYSE Alternext Paris since May 3rd 2012. For further information, please visit www.vexim.com
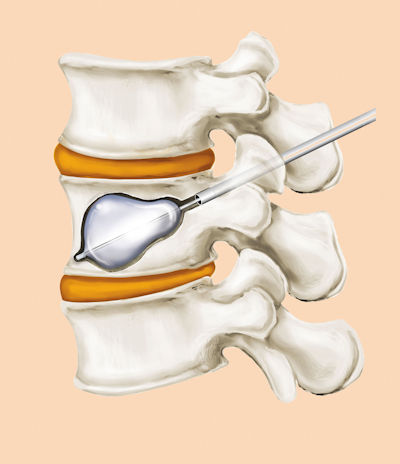
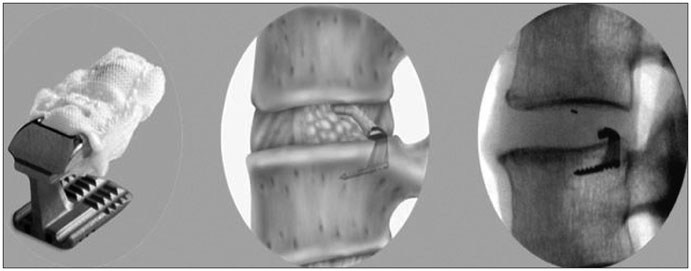
SpineJack®7, an innovative implant for treating Vertebral Compression Fractures
The SpineJack® is designed to restore a fractured vertebra to its original shape, restore the spinal column’s optimal anatomy and thus remove pain and enable the patient to recover their functional capabilities. Thanks to a specialized range of instruments, inserting the implants into the vertebra is carried out by minimally invasive surgery, guided by X-ray, in approximately 30 minutes, which is intended to enable the patient to be discharged shortly after surgery. The SpineJack® range consists of 3 titanium implants with 3 different diameters, thus covering 95% of vertebral compression fractures and all patient morphologies. SpineJack® technology benefits from the support of international scientific experts in the field of spine surgery and worldwide patent protection through to 2029.
Nom : VEXIM
Code ISIN : FR0011072602
Code mnémonique : ALVXM
1 Internal Vexim source.
2 Internal Vexim source.
3 Mehlman, Charles T., DiPasquale, Thomas G. Journal of Orthopaedic Trauma: August 1997 – Volume 11 – Issue 6 – pp 392-398 Radiation Exposure to the Orthopaedic Surgical Team During Fluoroscopy: “How Far Away Is Far Enough?”
4 http://en.vexim.com/professionals/scientific-and-clinical-communication/
5 Indicative date, subject to changes.
6 Founded in 2001 in Paris, Truffle Capital is a leading independent European private equity firm. It is dedicated to investing in and building technology leaders in the IT, life sciences and energy sectors. Truffle Capital manages €550m via FCPRs and FCPIs, the latter offering tax rebates (funds are blocked during 7 to 10 years). For further information, please visit www.truffle.fr and www.fcpi.fr.
7 This medical device is a regulated health product that, with regard to these regulations, bears the CE mark. Please refer to the Instructions for Use.
Contacts
VEXIM
Vincent Gardès, CEO
José Da Gloria, Chief Financial Officer
Tel.: +33 5 61 48 48 38
investisseur@vexim.com
or
PRESS RELATIONS
ALIZE RP
Caroline Carmagnol / Wendy Rigal
Tel.: +33 1 44 54 36 66
Tel.: +33 6 48 82 18 94
vexim@alizerp.com