June 1, 2017
DAYTON, Ohio – The Accelerant Venture Capital Fund recently approved investment in AMB Surgical to support the company’s development of a prototype for its Flyte Automated Growing Rod, a medical device that could revolutionize the way physicians treat juvenile scoliosis, lengthen limbs, and stabilize traumatic injuries.
The company founders bring decades of engineering experience to their work, as well as a personal mission to make such surgeries less traumatic for young patients.
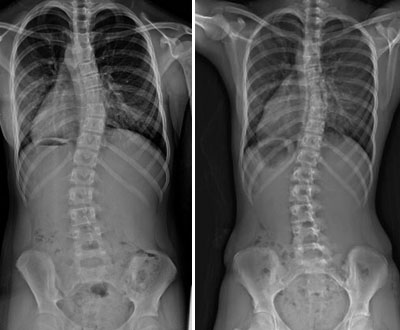
Founder Casel Burnett would know. His daughter was diagnosed with juvenile scoliosis as a young child and endured years of invasive surgeries as part of the treatment. Scoliosis, or curvature of the spine, can lead to life-long health issues if not corrected while the child is still growing. Watching his daughter undergo treatment motivated him to find a better way.
“AMB embodies the innovative spirit and hard work that characterize the Dayton Region,” said Roger Edwards, Vice President of Accelerant Venture Capital Fund, an initiative of the Dayton Development Coalition. “Their work has the potential to revolutionize the way these conditions are treated and spare young patients repeated, invasive procedures.”
Burnett is a mechanical engineer and group manager of production engineering at Toyota, and partnered with friend Tyson Ross, an electrical controls engineer and program manager at the U.S. Air Force Research Laboratory, to see how they could take the existing technology and improve it. Ross and Burnett are confident they have found the answer.
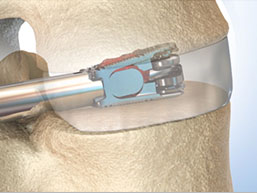
AMB uses patented technology to electronically control the extension of rods used in orthopedic surgeries. Current devices use manual adjustments, either by having caretakers, such as parents, use tools to adjust an external framework for limb extension, or, for scoliosis, through repeated back surgeries. A physician implants an adjustable rod along the child’s spine, and as the child grows, uses tools to lengthen the rod through additional surgeries to correct the curve.
Flyte would allow the physician to expand the rod remotely, without surgery, as the child grows, both cutting down on the number of surgeries and potentially accelerating the treatment. It would eliminate the need for parents to adjust external frames, as well as reduce the likelihood of missing adjustments.
The key to their technology is tiny gear box located in the rod that has a microchip and RFID reader to program adjustments and record information.
Dr. Michael Albert, Director or Orthopaedics at Dayton Children’s Hospital, called the product a “game changer” in this type of treatment.
“We have made significant progress in the safety and design of limb lengthening external fixator devices, but what has been lacking is an automated, safe and reliable device that removes the patient or family from making daily adjustments,” Dr. Albert wrote in a letter of support for AMB.
Ross said they plan to develop a prototype with the current round of funding, starting with a rod that would be used in external frames, then moving on to implanted rods for scoliosis treatment and other internal uses.
For physicians, the device would also provide valuable feedback they can’t access today. The same technology that allows the device to adjust, allows it to collect data on the patient’s progress. The company already holds patents on the technology, and the initial research and design have generated interest from world leaders in pediatric orthopedic surgery.
“How small can we go? That’s the real question,” Ross said. “There’s tremendous potential for this type of technology, and we are confident we can bring it to market.”
The product would need to undergo testing and approval from the Federal Drug Administration.
The investment marks the 10th investment for Accelerant, the region’s only institutional VC fund with resources ready to invest in local companies. AMB has attracted significant “sidecar” investment as well, as private local investors find increasing opportunities to invest in start-ups in the Dayton Region.
“In the last year, we’ve seen a real shift in funding for local start-ups,” Edwards said. “The support and investment of local ‘angel investors’ is vital for start-ups to flourish. It’s exciting to connect local entrepreneurs to investors in the Dayton Region so they can grow their businesses here.”
AMB’s technology has generated interest from outside the Dayton region, as well. Dan Sands, a 25-year healthcare industry executive, has identified and is retaining world-renowned surgeon advisors, subject matter experts in engineering, accredited investment sources, and potential strategic commercialization entities.
“The positive market interest for this technology has been extraordinary,” said Sands, CEO of D11, LLC, who has spent most of his career in Warsaw, Ind., a city known within the industry as the “Orthopaedic Capital.” “Flyte’s ability to give surgeons real-time biomechanical stress/strain data will allow more accurate and timely remote non-surgical adjustments. This represents a game changing advancement in orthopaedics and could lead to a new frontier in bionic implantable devices that improves the total cost and quality of care.”
AMB plans to engage The Ohio State University’s Center for Design and Manufacturing Excellence and other expert resources as part of its efforts to develop the initial productionready prototypes. The Ohio State center plans to leverage its expertise to facilitate design feasibility, component and systems development, prototyping, manufacturing scale, and workforce development. “Working with Ohio State’s CDME brings resources and expertise to the process at an early stage that is difficult to find elsewhere in the country,” Ross said.
As for the young girl who inspired this technology, Burnett’s daughter Ashley Mae is all grown up, working as a nurse, and in great health. She’s the name behind AMB.
“We started down this path to help her, and I hope the work we’ve done will give children an easier journey than she had,” Burnett said. “We’re grateful for the support we’ve found here in Dayton and are excited to move forward with this opportunity.”
Dayton Development Coalition (DDC) is the leading economic development organization for the 14- county Dayton region. Working closely with public and private regional partners, its mission is to retain, expand and recruit jobs. The DDC and the Dayton region ranked as the nation’s top metro for its size for economic development in 2008, 2009 and 2012 and runner-up in 2010, 2011, 2014 and 2015. With the State’s largest single-site employer in its backyard, the DDC also focuses on advocating for the critical missions at Wright-Patterson Air Force Base and supporting the work of the United States Air Force. The DDC was ranked as America’s top military community, and was presented with the 2015 Community Excellence Award from the Association of Defense Communities. It was designated as one of the ADC’s Great American Defense Communities in 2016 – a testament to the collaborative efforts in support of Wright-Patt, America’s #1 Air Force Base as ranked by the Air Force Times (2014). For any questions regarding the DDC, contact Shannon Joyce Neal at (937) 723-2047. For more information on AMB, contact Dan Sands, 574-527-1525, or dan.sands@outlook.com.
For any questions regarding the DDC, contact Shannon Joyce Neal at (937) 723-2047. For more information on AMB, contact Dan Sands, 574-527-1525, or dan.sands@outlook.com.