ATLANTA, GA–(Marketwired – Jun 12, 2017) – Medovex Corp. (NASDAQ: MDVX), a developer of medical technology products, today released the following open letter to shareholders:
Dear Fellow Shareholder,
Since my last letter, we have achieved great strides in the development of our flagship product, the DenerveX™ System. These efforts recently culminated in our official receipt of both ISO 13485 Certification and CE Mark approval for the DenerveX System allowing for the Company to now commence selling and marketing the DenerveX System in Europe and other countries that accept the CE Mark. Years in the making, this milestone event officially marks the company’s transition from a development stage company to a commercialization stage company.
Initial orders received:
Since receiving CE Mark, we have received orders for the DenerveX Kits and the DenerveX Pro-40 generators from TCB Distribution Center in Berlin, Germany, as well as orders from TCB Ortho, our Germany distributor and EDGE Medical in the Manchester, England.
First in human cases:
We are pleased to announce that three centers in the EU will perform our first in human cases for the DenerveX System. They include hospitals in Manchester, England; Stuttgart, Germany and Potsdam, Germany with committed initial DenerveX System Cases of ten patients at each center. These cases are scheduled to begin in July.
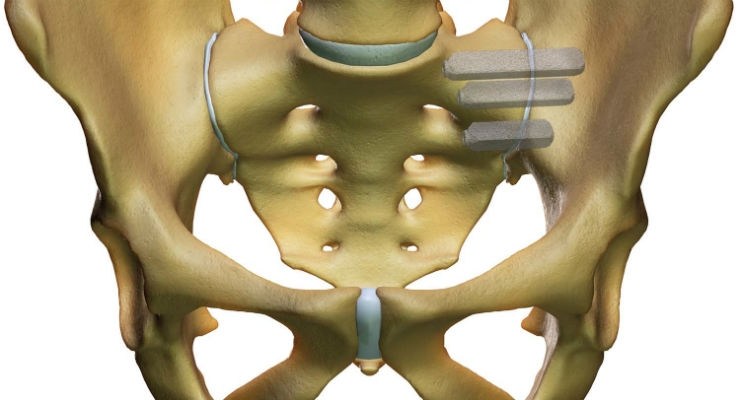
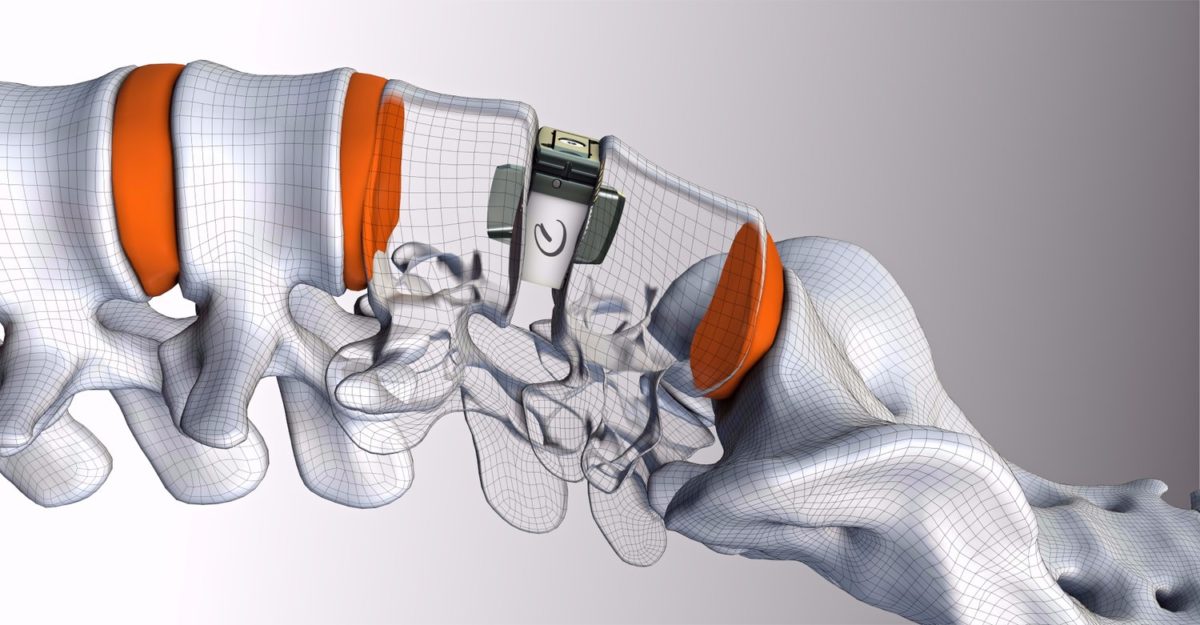
The DenerveX System is designed to provide longer term relief from debilitating pain associated with Facet Joint Syndrome (FJS). Lower back pain (LBP) remains the second most common cause of disability in the U.S., and affects approximately 10% of adults. Studies indicate that that 31% of lower back pain cases are attributed to FJS.
The DenerveX System consists of the single use DenerveX Kit containing the DenerveX Device, powered by the dedicated DenerveX Pro-40 generator. The system represents a highly disruptive product targeting a large and expanding market opportunity. Importantly, it was specifically designed by surgeons for surgeons, to be less invasive with faster recovery time than current surgical treatment options, while also providing for a longer lasting treatment solution and offering potential savings to the health care system.
A presentation published by Roger Chou, MD, and Associate Professor of Medicine at Oregon Health and Science University, indicated that lower back pain is the fifth most common reason for U.S. doctor office visits, and the second most common symptomatic reason. The presentation indicated that that there is an estimated greater than $100 billion dollars in total health care expenditures for LBP in the U.S. It further cited LBP as the most common cause for activity limitations in persons under the age of 45. The presentation further goes on to discuss the growing epidemic of Opioid addiction related to increased physician prescribing patterns for pain management. Our DenerveX System was specifically designed to address long term pain management seeking to alleviate, and or mitigate pain, in addition to potentially reducing dependence on pharmaceutical based remedy.
The EU launch scope and size:
The team has worked very effectively in securing twelve separate distribution agreements that cover 19 countries mostly in the EU, and a few limited countries in Asia Pacific and Latin America that accept the CE Mark. Our plans are to conduct a controlled pilot launch in the UK and Germany to validate and verify the elements of the launch plan by approaching the market with key opinion leaders in high volume centers. Our plan is to go “narrow and deep” with a few selected high volume key opinion leaders initially. Our pilot will include the UK and Germany followed by designated countries for a controlled launch managing risk while maximizing opportunity in a very sizable market in the EU. Our launch plans are complete and fully covering in scope for all sales, marketing and distribution areas.
NSpine-Launch Meeting-June 12-15, 2017:
This week, our team will be in attendance at The NSpine Main Conference in London, UK where we will be conducting a DenerveX System cadaver lab and work shop lecture with leading spine surgeon and advisory board member Dr. Vik Kapoor. The NSpine show will be an excellent meeting venue to meet with key surgeons. The UK market is very important to us since it will be a leading market for our initial entry into Europe on the heels of receiving the CE Mark.
As part of our European sales launch, I’d like to highlight a few objectives we are actively initiating. A key aspect of our sales initiative is obviously physician training and education. Our objective is to optimize physician training for positive patient treatment outcomes, procedural reproducibility and efficiency for the treatment of Facet Joint Syndrome with the DenerveX System. The DenerveX procedure is taught in a step-by-step reproducible industry standard method, using tools provided by Medovex and their highly skilled and experienced trainers through DenerveX lecture and observation of actual patient cases.
Launch and rollout physician training strategy:
We have planned a highly effective and historically proven customer training process used throughout the industry. We will offer peer-to-peer DenerveX training sessions for physicians at a “DenerveX University” designated training site in the EU. This advanced clinical training is a physician-led program to address any clinical questions regarding this innovative technology. These training physicians have been selected based on their passion and experience in the DenerveX approach.
As we transition from a development focused organization to a revenue generating company, I’m keenly aware that being a public company involves the management of not just our actual fundamental business, but also the public markets, compliance with trading exchanges, and investor communications.
With this in mind, I want to touch on the Company’s recently received Nasdaq deficiency letter, details of which may be found in a Form 8K filed with the SEC. I want you to know that we are taking the notification very seriously and have engaged a strategic consultant proficient in advising companies in similar situations to help us formulate and propose a plan to regain compliance. We have a hearing with Nasdaq on June 15th to discuss the matter. It is fully our intent to do whatever is necessary to comply and satisfy the matter to preserve our listing on the exchange.
Regarding the current valuation of the company and its shares, I am not satisfied. I believe our stock is significantly undervalued relative to other medical device companies, and especially in light of what many would consider a transformational event; receipt of both ISO Certification and CE Mark. I think it’s important to note that my team and I have real world experiencing in building businesses into profitable enterprises. I personally built my last company from a single office with just four employees, into two hundred and seventy offices located in four states, and employing over five hundred people. I am dedicated to doing the same here by taking our product and building a business that is expected to provide return on investment for all of our shareholders. Again, the DenerveX System was designed for surgeons by surgeons in an effort to address long term pain management.
My personal conviction and commitment, along with other insiders, can be no better evidenced than by reviewing an already long and growing list of repeated insider purchases memorialized in Form 4’s filed with the SEC. You will note that I just personally bought additional shares, along with the Company’s co-founder, since the announcement of our receipt of ISO Certification and CE Mark approval. In addition, board member and DenerveX Device inventor Dr. Scott Haufe also made significant purchases in recent months. I believe it is important that we lead by example, putting our own money where our mouth is. Recent filings can only be seen for what they are, again an affirmation of our commitment and belief in both our team and the opportunity.
In closing, while I want to thank all of you as our shareholders, I want to especially thank the entire Medovex team. Together, we’ve taken a concept to a commercial product and are now entering a growth stage that we’ve all been working towards for the last two years. While we continue to have a lot of work ahead of us as we focus on driving adoption and revenue, I have confidence that we will excel in achieving our future goals, much like we did in getting here today.
“Better living through better medicine” is not just a slogan; it’s a philosophy that management and our board are committed to seeing through in the form of innovative new products like our DenerveX Device. Thank you again for your valued support.
Kind regards,
Jarrett Gorlin
Chief Executive Officer
About Medovex
Medovex was formed to acquire and develop a diversified portfolio of potentially ground breaking medical technology products. Criteria for selection include those products with potential for significant improvement in the quality of patient care combined with cost effectiveness. The Company’s first pipeline product, the DenerveX System, is intended to provide long lasting relief from pain associated with facet joint syndrome at significantly less cost than currently available options. The DenerveX System is CE Marked while not yet FDA approved. To learn more about Medovex Corp., visit www.medovex.com.
Safe Harbor Statement
Certain statements in this press release constitute “forward-looking statements” within the meaning of the federal securities laws. Words such as “may,” “might,” “will,” “should,” “believe,” “expect,” “anticipate,” “estimate,” “continue,” “predict,” “forecast,” “project,” “plan,” “intend” or similar expressions, or statements regarding intent, belief, or current expectations, are forward-looking statements. While the Company believes these forward-looking statements are reasonable, undue reliance should not be placed on any such forward-looking statements, which are based on information available to us on the date of this release. These forward looking statements are based upon current estimates and assumptions and are subject to various risks and uncertainties, including without limitation those set forth in the Company’s filings with the Securities and Exchange Commission (the “SEC”), not limited to Risk Factors relating to its patent business contained therein. Thus, actual results could be materially different. The Company expressly disclaims any obligation to update or alter statements whether as a result of new information, future events or otherwise, except as required by law.