New Orleans, Louisiana – July 25, 2017 – LongueVue Capital (“LVC”) is pleased to announce it has partnered with management to acquire Zavation Medical Products (“Zavation” or the “Company”) and provide capital for growth. Zavation is a designer and manufacturer of high quality spinal implants, instruments, and biologics. Zavation expands LVC’s healthcare portfolio and is the second medical device business LVC has partnered within the past 18 months.
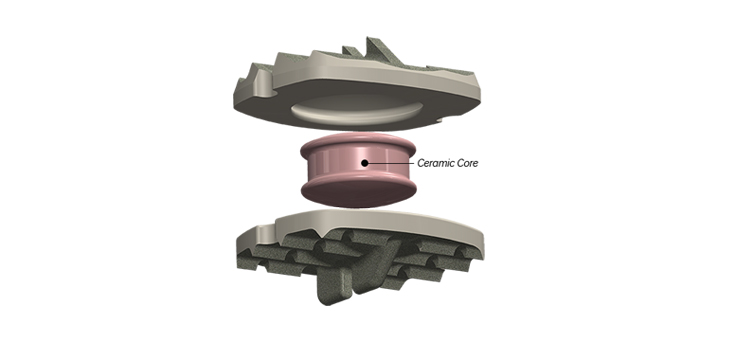
Based in Jackson, MS, Zavation designs, engineers, and manufactures a portfolio of spinal hardware covering key areas including thoracolumbar, cervical, interbody fusion, and minimally invasive surgery. Founded in 2010 with its first sale in 2012, Zavation has experienced exceptional growth and created a national network of 100+ distributors across approximately 35 states. The Company has commercialized over 10 product families since inception, with 10 additional novel products expected to launch over the next two years. Zavation operates a 24,000 square foot vertically integrated facility with approximately 50 employees.
“Zavation has demonstrated exceptional growth and is a perfect cornerstone to our expanding healthcare portfolio. This is our fourth healthcare platform investment and our third in the past 18 months,” said Ryan Nagim, Principal and deal lead at LVC. “Furthermore, we believe the Company is at an inflection point and has the opportunity to become a market leader in the spinal implant sector, as management has shown an incredible ability to execute. As such, we look forward to our partnership with Zavation’s management team and driving significant growth, both organically and through acquisitions of unique technologies.”
“Zavation fits well within LVC’s investment strategy,” added Rick Rees, Founder and Managing Partner of LVC. “We love partnering with entrepreneur owned businesses at inflection points that have proven management teams willing to invest meaningfully alongside LVC. The Company’s rapid growth is a testament to management’s ability to capitalize on compelling demographics and other industry tailwinds favoring smaller, more agile, and customer focused OEMs. Lastly, although we are geographically agnostic, it is always great to partner with entrepreneurs in our back yard, the Gulf South. As such, we are incredibly excited to partner with management and welcome Zavation into the LVC family.”
“The management team at Zavation chose LVC due to their entrepreneur-friendly investment approach to value creation and their knowledge and expertise in growing middle market companies at inflection points like ours. With a shared vision and culture, we are confident Zavation will remain focused on its core values of excellent customer service and quality products,” said Jeffrey Johnson, President and CEO of Zavation. “LVC’s financial and operational resources will allow us to introduce new products, meet and exceed distributor and surgeon expectations, expand our market presence, and take market share at an even faster and more deliberate speed. We are excited for our Company, our distributors, our surgeons, and our LVC partners.”
Robert W. Baird & Co.’s healthcare team, led by Robert Andrews and Manish Gupta, served as exclusive financial advisor to LVC on the transaction. Abacus Finance Group, LLC provided the senior debt financing to Zavation Medical Products in support of the transaction. LVC’s legal counsel was provided by McGuireWoods, LLP.
To learn more information about Zavation and its products, visit www.zavation.com
About LongueVue Capital:
LongueVue Capital is a private equity firm focused on making situation-driven, value-oriented equity and debt investments in lower middle market companies (up to $150 million in annual revenue) to support buy-outs, recapitalizations, acquisitions and growth. LVC currently has $425 million under management across two funds. Since its formation in 2001, LVC has made successful investments in a wide variety of industries, including healthcare, business services, transportation and logistics, energy services, and niche manufacturing. LVC is based in New Orleans with additional offices in New York and Salt Lake City. For more information, please visit www.lvcpartners.com.
For inquiries, please contact lvc@lvcpartners.com or call 504-293-3600.