SAN JOSE, Calif., Sept. 25, 2017 /PRNewswire/ — SI-BONE, Inc., an innovative medical device company which pioneered the use of the iFuse Implant System® (iFuse), a triangular-shaped minimally invasive surgical (MIS) device indicated for fusion for certain disorders of the sacroiliac (SI) joint, announced the publication of one-year results from iMIA (iFuse Implant System Minimally Invasive Arthrodesis; ClinicalTrials.gov ID NCT01741025). iMIA is a randomized controlled trial, conducted at 9 hospitals in 4 countries in Europe, that assessed the safety and effectiveness of SI joint fusion with the iFuse Implant compared to conservative management (CM) in patients with chronic SI joint dysfunction. This Level 1 study was published in Pain Physician, the official journal of the American Society of Interventional Pain Physicians and titled 1-Year Results of a Randomized Controlled Trial of Conservative Management vs. Minimally Invasive Surgical Treatment for Sacroiliac Joint1. The study showed that for patients with chronic low back pain (LBP) due to certain types of SI joint dysfunction, minimally invasive SI joint fusion with the iFuse Implant was safe and more effective than CM in relieving pain, reducing disability, and improving patient function and quality of life at one year. The full article can be found at the following link: http://www.painphysicianjournal.com/current/pdf?article=NDYwOQ%3D%3D
The study included 109 subjects enrolled between June, 2013 and May, 2015 and follow-up for this publication extends through October, 2016. Mean age was 48.1 years, 75 subjects (72.8%) were women and mean SI joint pain duration was 4.7 years. Most (72.8%) had undergone prior SI joint steroid injections, a minority (16.5%) had had prior radiofrequency ablation and about 1/3 (35.9%) had undergone prior lumbar fusion, a known risk factor for SI joint pain. Patients in the surgical arm of the study were treated with the iFuse Implant, which has been commercially available in the United States since 2009.
As shown in the figures below, at one year, mean LBP improved by 41.6 VAS points (0 – 100 VAS pain scale) in the SI joint fusion group compared to 14.0 points in the CM group (Figure 1), and the mean Oswestry Disability Index (ODI) improved by 25.0 points in the SI joint fusion group compared to 8.7 points in the CM group (Figure 2). Also, mean improvements in leg pain and EQ-5D-3L were large after SI joint fusion and superior to those after CM. CM subjects were allowed to cross over to SI joint fusion after six months and subjects who crossed over to surgical treatment had no pre-crossover improvement in pain and ODI scores. After crossover, improvements in most measures were as large as those patients originally assigned to SI joint fusion.
“It’s been most gratifying to be a part of this important trial to help identify the value and benefits of the use of the iFuse Implant for SI joint patients who no longer benefit from conservative therapies,” said Bengt Sturesson, MD, from Ängelholm Hospital, Ängelholm, Sweden and one of the study authors. “The one-year results from iMIA clearly show consistent outcomes with the previously published U.S. RCT, INSITE, thus further validating the applicability of the iFuse Implant to patients across a broad spectrum of clinical practitioners.”
Aaron Calodney, MD of Texas Spine & Joint Hospital in Tyler, TX said, “as a member and past president of the American Society of Interventional Pain Physicians, I am delighted to see such a high-quality study be published in the Pain Physician journal. In addition, I continue to be impressed with both the quantity and quality of the clinical evidence being generated that clearly separates the iFuse Implant from all other SI joint surgical options.”
About SI joint dysfunction
The SI joint has been attributed as a source of pain in 15-30 percent of patients with chronic low back pain2-5, and in up to 43 percent of patients with new onset or persistent low back pain after lumbar fusion.6 Like all other major joints, the SI joint can be injured or degenerate, which can cause debilitating pain in the lower back, buttocks and legs. Simple movements such as standing up, sitting down, stepping up or down, bending and lifting, walking, or even sleeping or sitting on the affected side can provoke a symptomatic SI joint.
SI joint dysfunction is often misdiagnosed and the resulting pain can be incorrectly attributed to other causes. Not all healthcare providers evaluate the SI joint and many patients do not know to ask about it. While not commonly diagnosed, SI joint disorders can be identified when a patient points to their source of pain directly over the posterior superior iliac spine (PSIS) known as the Fortin Finger Test, combined with a number of positive provocative maneuvers to stress the SI joint and elicit the pain, followed by image-guided diagnostic injections to confirm the diagnosis.
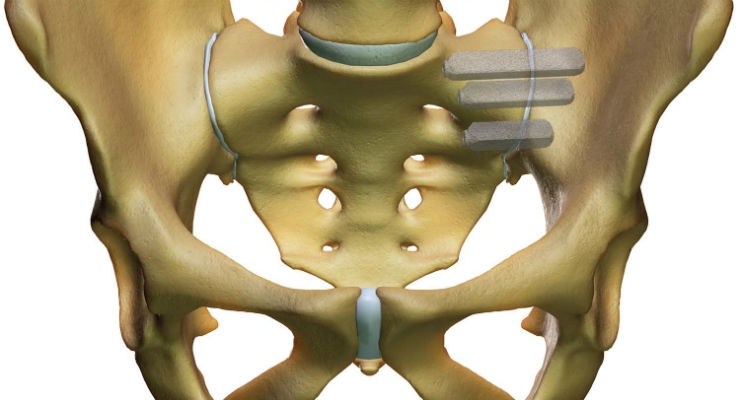
The other major joints in the human body, such as knees, hips, ankles and shoulders, have specialized device-based surgical solutions. The SI joint is the largest and the last of eight major joints in the human body to have a proven surgical solution. The iFuse Implant™ was designed specifically to withstand the extreme forces resulting from load-bearing and the unique rotational and translational motion of the SI joint referred to as nutation, and is supported by more than 50 peer-reviewed publications including two Level 1 randomized controlled trials.
About SI-BONE, Inc.
SI-BONE, Inc. (San Jose, California) is a leading medical device company that has developed the iFuse Implant System, a proprietary minimally invasive surgical implant system to fuse the sacroiliac joint to treat common disorders of the joint that can cause lower back pain. Patients with certain types of sacroiliac joint dysfunction experience pain that can be debilitating. SI-BONE believes that the sacroiliac joint is the last of the eight major joints in the human body to have a proven surgical treatment and that the iFuse Implant, first FDA-cleared in 2009, is the only device for treatment of SI joint dysfunction supported by significant published clinical evidence, including level 1 trials, showing safety and durable effectiveness, including providing lasting pain relief.
The iFuse Implant System is intended for sacroiliac fusion for conditions including sacroiliac joint dysfunction that is a direct result of sacroiliac joint disruption and degenerative sacroiliitis. This includes conditions whose symptoms began during pregnancy or in the peripartum period and have persisted postpartum for more than 6 months. There are potential risks associated with the iFuse Implant System. It may not be appropriate for all patients and all patients may not benefit. For information about the risks, visit: www.si-bone.com/risks
SI-BONE and iFuse Implant System are registered trademarks of SI-BONE, Inc. ©2017 SI-BONE, Inc. All Rights Reserved. 9760.092517
|
1. Dengler J, Kools D, Pflugmacher R, et al. 1-Year Results of a Randomized Controlled Trial of Conservative Management vs. |
SOURCE SI-BONE, Inc.