CARLSBAD, Calif., Nov. 01, 2017 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ:SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced the limited commercial launch and completion of initial surgeries for its OsteoStrand™ Demineralized Bone Fibers. The first implantations were completed by Dr. Khalid Abbed, Vice Chair of Neuro Surgery and Director of Minimally Invasive Spine Surgery at Yale School of Medicine and orthopedic spine surgeon Michael W. Cluck, M.D., PhD, at the Bay Area Spine Care in San Jose, CA.
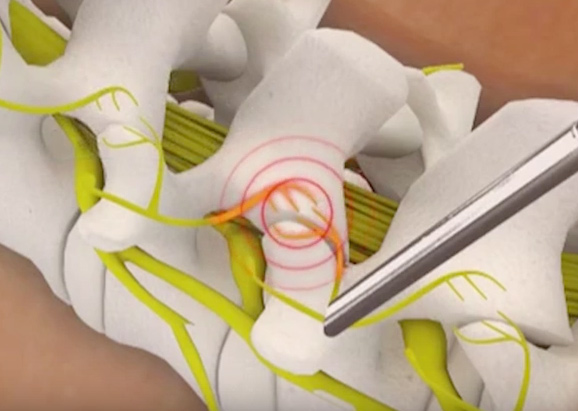
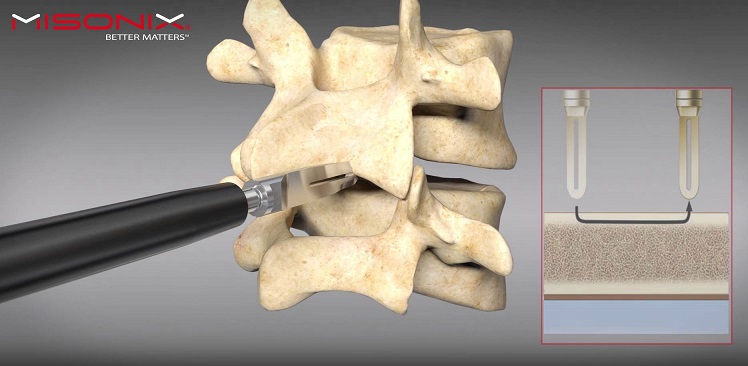
OsteoStrand fibers are the latest innovation in SeaSpine’s demineralized bone matrix (DBM) product family, which facilitate and aid in fusion. OsteoStrand fibers provide 100% demineralized bone fibers to maximize the osteoinductive content while providing a vastly improved conductive matrix. These fibers were developed through a disciplined R&D process that evaluated a variety of fiber geometries to deliver intraoperative handling and controlled expansion, to facilitate surgical placement, to maintain surgical position and to allow the fibers to better fill the surgical defect with the overriding goal to improve fusion potential.
“The handling properties of OsteoStrand Demineralized Bone Fibers are excellent; it is easily compressible and expands to fill the surgical defect upon implantation,” said Dr. Cluck. “The handling and 100% demineralized bone composition give me a high level of confidence for my fusion procedures.”
Dr. Khalid Abbed added, “The handling properties of OsteoStrand Demineralized Bone Fibers are excellent, and improved compared to other 100% DBM products that I have used in my practice. I believe this tissue product could lead to significant cost savings for my hospital and I look forward to evaluating the OsteoStrand Plus with Accell Bone Matrix.”
“OsteoStrand fibers will serve as a development platform for continued innovation in our orthobiologics portfolio and will further strengthen our top-three position in the U.S. DBM market while also providing operational leverage through our Irvine manufacturing facility,” said Keith Valentine, Chief Executive Officer and President of SeaSpine. “We plan to launch the OsteoStrand Plus Demineralized Bone Fibers with our proprietary Accell™ Bone Matrix on a limited basis in early 2018. We believe these product offerings deliver clinical value as payors and hospitals seek more cost effective orthobiologic solutions.”
About SeaSpine
SeaSpine (www.seaspine.com) is a global medical technology company focused on the design, development and commercialization of surgical solutions for the treatment of patients suffering from spinal disorders. SeaSpine has a comprehensive portfolio of orthobiologics and spinal implants solutions to meet the varying combinations of products that neurosurgeons and orthopedic spine surgeons need to perform fusion procedures on the lumbar, thoracic and cervical spine. SeaSpine’s orthobiologics products consist of a broad range of advanced and traditional bone graft substitutes that are designed to improve bone fusion rates following a wide range of orthopedic surgeries, including spine, hip, and extremities procedures. SeaSpine’s spinal implant portfolio consists of an extensive line of products to facilitate spinal fusion in minimally invasive surgery (MIS), complex spine, deformity and degenerative procedures. Expertise in both orthobiologic sciences and spinal fusion hardware product development helps SeaSpine to offer its surgeon customers a complete solution to meet their fusion requirements. SeaSpine currently markets its products in the United States and in over 30 countries worldwide.
Forward-Looking Statements
SeaSpine cautions you that statements included in this news release that are not a description of historical facts are forward-looking statements that are based on the Company’s current expectations and assumptions. Such forward-looking statements include, but are not limited to, statements relating to: the potential benefits of the OsteoStrand Demineralized Bone Fibers product, including to improve fusion; the ability of the OsteoStrand Demineralized Bone Fibers to strengthen SeaSpine’s top-three position in the U.S. DBM market and provide operational leverage; the timing of launch of the OsteoStrand Plus Demineralized Bone Fibers product in early 2018; and the potential for new product offerings to clinical value to payors and hospitals. Among the factors that could cause or contribute to material differences between our actual results and the expectations indicated by our forward-looking statements are risks and uncertainties that include, but are not limited to: the ability of newly launched products to perform as designed and intended and to meet the clinical needs of surgeons and patients; the limited clinical experience supporting the commercial launch of new products and the risk that such products may require substantial additional development activities, which could introduce unexpected expense and delay; the lack of long-term clinical data supporting the safety and efficacy of the Company’s products; and other risks and uncertainties more fully described in our news releases and periodic filings with the Securities and Exchange Commission. The Company’s public filings with the Securities and Exchange Commission are available at www.sec.gov.
You are cautioned not to place undue reliance on forward-looking statements, which speak only as of the date when made. SeaSpine does not intend to revise or update any forward-looking statement set forth in this news release to reflect events or circumstances arising after the date hereof, except as may be required by law.
Investor Relations Contact
Lynn Pieper
(415) 937-5402
ir@seaspine.com