September 07, 2016
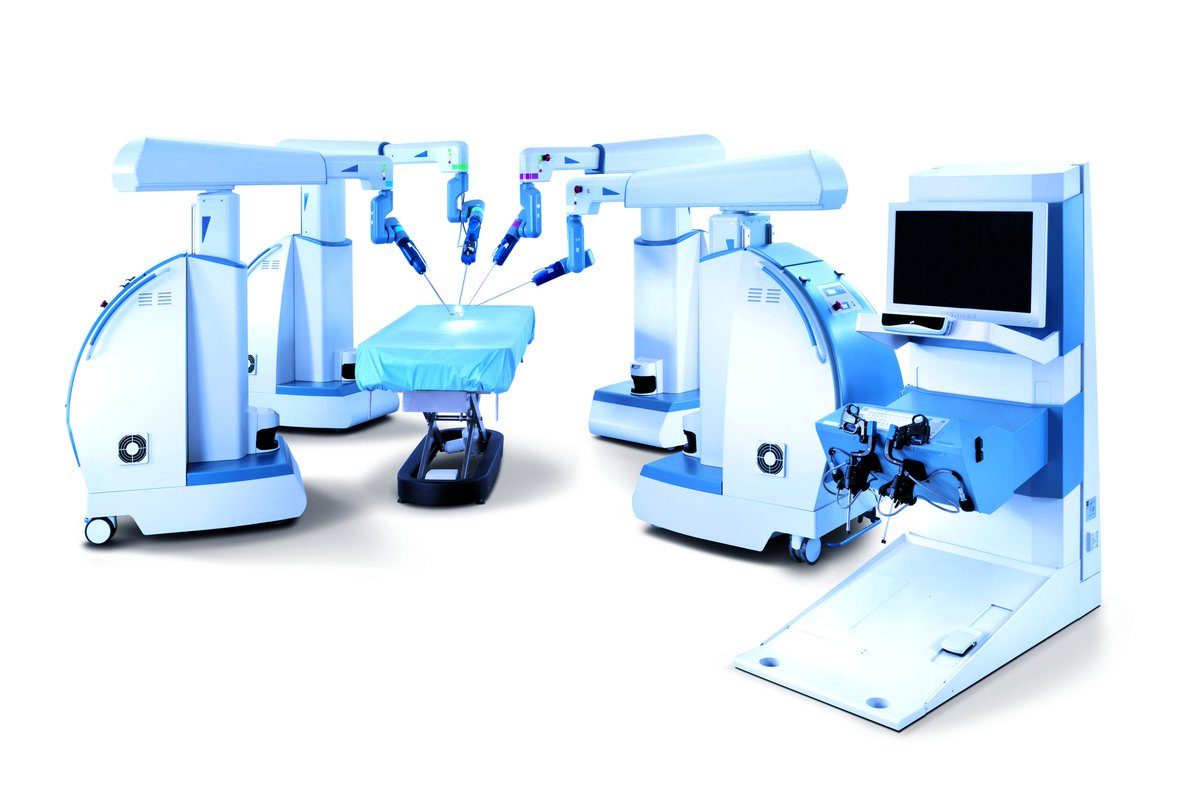
RESEARCH TRIANGLE PARK, N.C.–(BUSINESS WIRE)–TransEnterix, Inc. (NYSE MKT: TRXC), a medical device company that is pioneering the use of robotics to improve minimally invasive surgery, today announced the launch of a new brand identity for the ALF-X Robotic Surgical System, which henceforth will be known as The Senhance™ Surgical Robotic System (“Senhance”).
“We are pleased to announce the new brand identity for our multiport surgical robotic system,” said Todd M. Pope, President and CEO of TransEnterix. “This is a pivotal point in our growth strategy and we believe Senhance speaks directly to the benefits experienced with our system.”
Mr. Pope continued: “Our goal is to advance minimally invasive surgery and the tools surgeons have at their disposal to provide patients with the best possible care while also providing a more attractive value proposition to the hospital. Senhance is designed to enhance laparoscopic surgery and specifically empowers the senses of the surgeon in ways that were never previously possible. The security of haptics and the convenience of eye sensing camera control are meaningful ways that technology can provide a further extension of the surgeon’s skill.”
The new logo, evocative of a thumb-print combined with circuitry, is a representation of the strong connection that Senhance provides between the surgeon and technology.
Senhance carries the CE Mark for use in general surgery, gynecology, urology and thoracic surgery. TransEnterix is actively preparing a submission for U.S. FDA Clearance for Senhance.
About TransEnterix
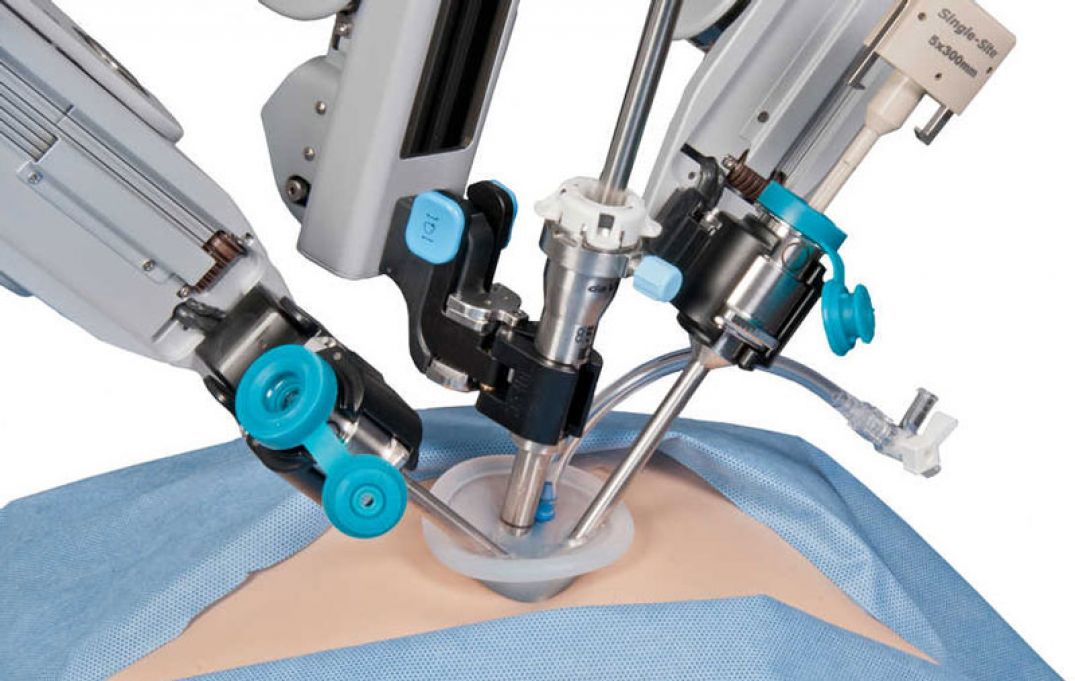
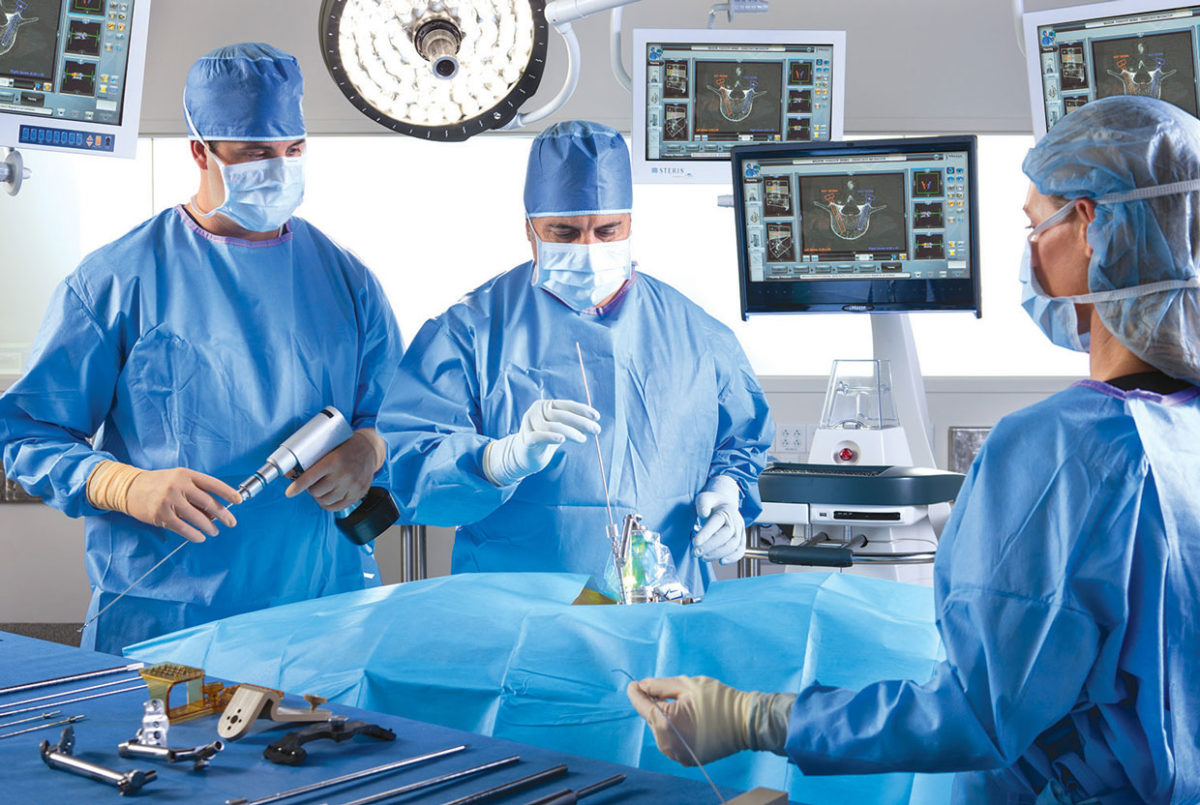
TransEnterix is a medical device company that is pioneering the use of robotics to improve minimally invasive surgery by addressing the clinical and economic challenges associated with current laparoscopic and robotic options. The company is focused on the commercialization of the Senhance Surgical Robotic System, a multi-port robotic system that brings the advantages of robotic surgery to patients while enabling surgeons with innovative technology such as haptic feedback and eye sensing camera control. The company is also developing the SurgiBot™ System, a single-port, robotically enhanced laparoscopic surgical platform. The Senhance Surgical Robotic System has been granted a CE Mark but is not currently available for sale in the United States. For more information, visit the TransEnterix website at www.transenterix.com.
Forward Looking Statements
This press release includes statements relating to The Senhance™ Surgical Robotic System and the SurgiBot™ System and our current regulatory and commercialization plans for these products. These statements and other statements regarding our future plans and goals constitute “forward looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, and are intended to qualify for the safe harbor from liability established by the Private Securities Litigation Reform Act of 1995. Such statements are subject to risks and uncertainties that are often difficult to predict, are beyond our control and which may cause results to differ materially from expectations, including whether Senhance speaks directly to the benefits both surgeons and patients experience with our Senhance system; whether Senhance is designed to enhance laparoscopic surgery and specifically empower the senses of the surgeon in ways never before provided. For a discussion of the risks and uncertainties associated with TransEnterix’s business, please review our filings with the Securities and Exchange Commission (SEC), including our Annual Report on Form 10-K filed on March 3, 2016 and our other filings we make with the SEC. You are cautioned not to place undue reliance on these forward looking statements, which are based on our expectations as of the date of this press release and speak only as of the origination date of this press release. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise.
Contacts
For TransEnterix, Inc.
Investor Contact:
Mark Klausner, +1 443-213-0501
invest@transenterix.com
or
Media Contact:
(For EU) Conrad Harrington, +44 (0)20 3178 8914
(For US) Hannah Dunning, +1 415-618-8750
TransEnterix-SVC@sardverb.com