March 15, 2017
SAN DIEGO–(BUSINESS WIRE)–DJO Global, Inc. (“DJO” or the “Company”), a leading global provider of medical technologies designed to get and keep people moving, today announced that its Exprt® Revision Hip portfolio received market clearance by the U.S. Food and Drug Administration. Exprt® Revision Hip is the latest addition to DJO Global’s Exprt® portfolio – a platform defying conventional approaches to total joint implants by improving outdated designs, focusing on patient outcomes and reinventing traditional price points.
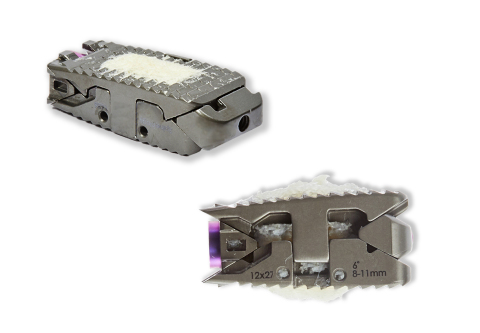
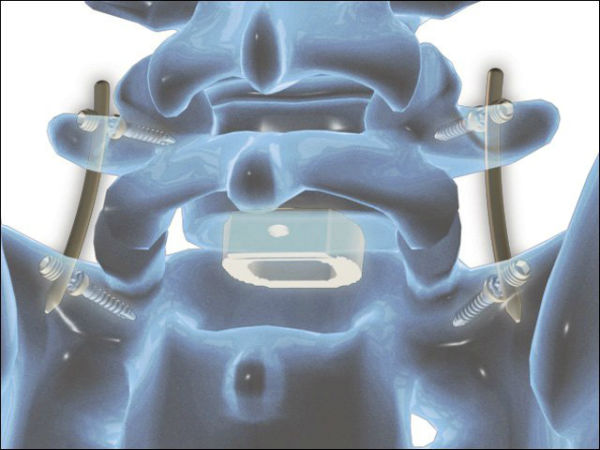
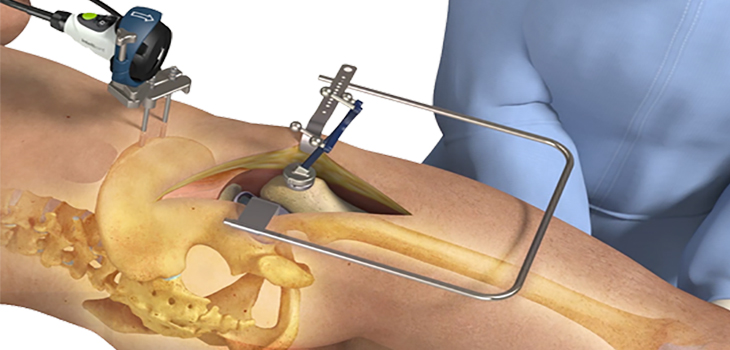
The 12,000 surgeons expected to attend this year’s Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS booth #1733) in San Diego, CA will have an opportunity to preview the full-line, modular femoral stem inspired by the clinical success of Wagner-style predecessors. The system’s intuitive design and premium quality is based on extensive research and development that redefines revision total hip arthroplasty by offering an anatomically inspired design that has an emphasis on efficiency and strength – all for 40-70% of the price of comparable revision hip systems.
“One of the biggest challenges our healthcare system encounters is introducing modern technologies at increased price points. The latest addition to the Exprt portfolio signifies our deep commitment to not only clinical outcomes, but both surgical and cost efficiencies,” said Mark Russell, Senior Vice President of the Surgical division of DJO Global.
The two-tray revision system represents an 80-90% reduction in instruments compared to competitive systems. Streamlined instrumentation translates into less money and time spent on sterilization, less overall time in the operating suite, and less storage space – appropriate for both hospital and ASC settings.
Exprt Revision Hip was developed in partnership with industry leading physicians. Michael Taunton, M.D. at the Mayo Clinic and design team member of the Exprt Platform was one of the first surgeons to implant the device. “Having a thoughtful approach to introducing new technology in today’s healthcare landscape will drive shifts in the market place. We designed DJO Global’s Exprt Revision Hip and Knee Systems to challenge conventional approaches to revision arthroplasty. These systems allow me to do what I used to think wasn’t possible – treat my patients with a high-quality implant with improved surgical efficiency and reproducible outcomes while simultaneously being economically responsible.”
For more information on the Exprt portfolio – including Revision Hip and Knee – please visit: www.exprtprecision.com
About DJO Global
DJO Global is a leading global provider of medical technologies designed to get and keep people moving. The Company’s products address the continuum of patient care from injury prevention to rehabilitation after surgery, injury or from degenerative disease, enabling people to regain or maintain their natural motion. Its products are used by orthopaedic specialists, spine surgeons, primary care physicians, pain management specialists, physical therapists, podiatrists, chiropractors, athletic trainers and other healthcare professionals. In addition, many of the Company’s medical devices and related accessories are used by athletes and patients for injury prevention and at-home physical therapy treatment. The Company’s product lines include rigid and soft orthopaedic bracing, hot and cold therapy, bone growth stimulators, vascular therapy systems and compression garments, therapeutic shoes and inserts, electrical stimulators used for pain management and physical therapy products. The Company’s surgical division offers a comprehensive suite of reconstructive joint products for the hip, knee and shoulder. DJO Global’s products are marketed under a portfolio of brands including Aircast®, Chattanooga, CMF™, Compex®, DonJoy®, ProCare®, DJO® Surgical, Dr. Comfort® and Exos™. For additional information on the Company, please visit www.DJOglobal.com.
Safe Harbor Statement
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Such statements relate to, among other things, the Company’s expectations for the success of the Exprt® product portfolio. The words “believe,” “will,” “should,” “expect,” ”target,” “intend,” “estimate” and “anticipate,” variations of such words and similar expressions identify forward-looking statements, but their absence does not mean that a statement is not a forward-looking statement. These forward-looking statements are based on the Company’s current expectations and are subject to a number of risks, uncertainties and assumptions, many of which are beyond the Company’s ability to control or predict. The Company undertakes no obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise. The important factors that could cause the results of the Exprt Hip products and other Exprt portfolio products to differ significantly from those expressed or implied by such forward-looking statements include, but are not limited to: the complexities and uncertainties associated with the development of new products; the uncertainties associated with acceptance of the new products by surgeons and patients; new product introduction and other business strategies relative to our Surgical Implant segment; the continued growth of the markets the Company addresses and any impact on these markets from changes in global economic conditions; the impact of potential reductions in reimbursement levels and coverage by Medicare and other governmental and commercial payors; the Company’s highly leveraged financial position; the Company’s ability to successfully develop, license or acquire, and timely introduce and market new products or product enhancements; risks relating to the Company’s international operations; resources needed and risks involved in complying with government regulations and government investigations; the availability and sufficiency of insurance coverage for pending and future product liability claims; and the effects of healthcare reform, Medicare competitive bidding, managed care and buying groups on the prices of the Company’s products. These and other risk factors related to DJO are detailed in the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, filed with the Securities and Exchange Commission on March 25, 2016. Many of the factors that will determine the outcome of the subject matter of this press release are beyond the Company’s ability to control or predict.
Contacts
DJO Investor/Media Contact:
DJO Global, Inc.
David Smith
SVP and Treasurer
760-734-3075
ir@djoglobal.com