BOCA RATON, Fla., Aug. 9, 2017 /PRNewswire/ — Hip Innovation Technology, LLC (HIT), a medical device company developing innovative orthopedic device solutions to advance the quality of life and quality of care for patients, today announces the initiation of a Multi-Center Prospective Study in Primary Total Hip Arthroplasty for its lead hip replacement system, the HRS. Total hip arthroplasty is commonly referred to as total hip replacement.
The objective of the clinical trial is to evaluate the effectiveness and safety of the HRS hip replacement system in 100 – 120 patients receiving a total hip arthroplasty (THA). Effectiveness will be evaluated using clinical, radiologic, radiostereometric and patient-reported outcomes. Safety will be assessed through the collection of device-related adverse events. Patient quality of life metrics will also be closely monitored.
“The HRS is a unique hip implant design that we believe represents breakthrough technology and a significant advancement for patients requiring total hip arthroplasty,” said George Diamantoni, Hip Innovation Technology’s Co-Founder and Chief Executive Officer. “We look forward to confirming potential differentiating clinical benefits including hip stability at extended ranges of motion, reduced risk of device dislocation and greater latitude for placement of hip components relative to the current designs.”
The company has extensively tested the HRS in over 80 standard and unique pre-clinical experiments to assess the product safety and clinical benefits anticipated by the unique system design.
“The bench level data compiled for the HRS is more extensive than any that I have reviewed for currently marketed hip implant systems,” said Thomas Turgeon, MD, Chief Medical Officer of the Orthopaedic Innovation Centre, and Orthopaedic Surgeon at Concordia Joint Replacement Group. “I am impressed with the unique system design. I look forward to assessing its clinical performance, and am hopeful that many patients will benefit from this novel device.”
Total hip replacements are one of the most effective ways to reduce joint pain and improve functioning for patients with advanced hip problems. During the 2015 calendar year, approximately 324,000 surgeries were performed in the U.S. and 50,000 in Canada.
About Hip Innovation Technology, LLC
Headquartered in Boca Raton, Florida, Hip Innovation Technology was formed in 2011 to provide market-leading orthopedic device solutions that advance the quality of life and quality of care for patients. In partnership with healthcare professionals worldwide, our goal is to identify unmet clinical need, then design, manufacture and ultimately market innovative orthopedic reconstructive and related surgical product solutions.
About the HRS
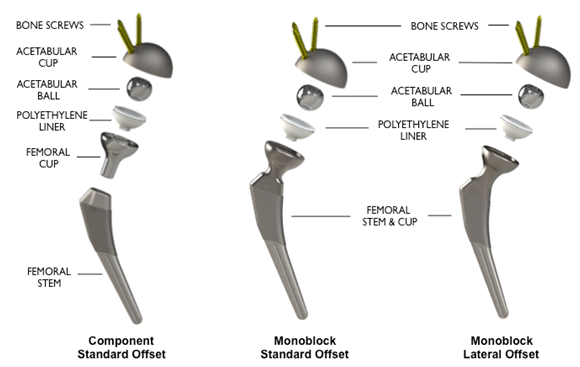
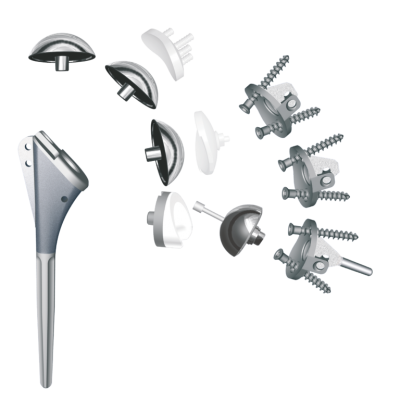
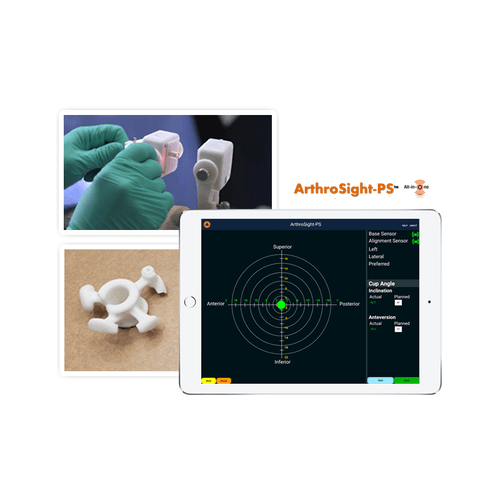
The HRS is a Metal-on-Polyethylene reverse geometry hip prosthesis designed to improve stability at extended ranges of motion and reduce the risk of dislocation. Like most conventional systems, the HRS consists of a femoral stem, an acetabular cup and a cobalt-chrome ball that articulates within a polyethylene liner. Unlike other systems, the ball sits on the acetabular cup instead of the femoral stem, and the polyethylene liner is attached to a femoral cup, which attaches to the femoral stem, instead of the polyethylene liner being attached to the acetabular cup. Despite this technological difference, the center of rotation of the HRS is similar to a normal physiological hip or a well-positioned Total Hip Arthroplasty. The advanced HRS implant design may provide greater range of motion in all planes with enhanced hip stability while significantly minimizing the risk of dislocation. In addition, the HRS may provide minimal postoperative restrictions and reduce the need for currently required durable medical equipment such as abduction pillows, elevated toilet seats and shower chairs. Importantly, the HRS also provides variability of component placement including higher abduction angles and anteversion of the acetabular cup. The femoral cup articulates around the acetabular ball and overlaps with the acetabular cup as the hip undergoes flexion-extension, abduction-adduction and internal-external rotation. This forgiving design compensates for suboptimal component positioning which likely provides benefits such as extended range of motion, hip stability and reduced likelihood of impingement. Simply stated, the HRS appears to uncouple the relationship between component placement, wear and stability. The unique implant design of the HRS provides optimal surface area contact between the acetabular ball and femoral cup, which may eliminate edge loading. Elimination of edge loading may provide benefits that include reduced high-contact stresses, decreased implant wear and uniform wear, which minimizes generation of wear debris and associated concerns related to osteolysis.
For more information, visit www.hipinnovationtechnology.com.
Cautionary Statement Regarding Forward-Looking Statements
This news release may contain forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include, but are not limited to, statements concerning Hip Innovation Technology’s expectations, plans, prospects, and product and service offerings, including new product launches and potential clinical successes. Such statements are based upon the current beliefs and expectations of management and are subject to significant risks and uncertainties that could cause actual outcomes and results to differ materially. Hip Innovation Technology disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. Accordingly, such forward-looking statements speak only as of the date made. Readers of this news release are cautioned not to place undue reliance on these forward-looking statements, since, while management believes the assumptions on which the forward-looking statements are based are reasonable, there can be no assurance that these forward-looking statements will prove to be accurate. This cautionary statement is applicable to all forward-looking statements contained in this news release.
Contact:
George Diamantoni, CEO
Hip Innovation Technology
InvestorRelations@HIT-IRH.com
Media:
Kara Golub
JFK Communications, Inc.
(609) 456-0822
kgolub@jfkhealth.com
SOURCE Hip Innovation Technology, LLC