NEW YORK, Aug. 31, 2017 /PRNewswire/ — MediPoint: Partial Knee Replacement – South America Analysis and Market Forecasts
Summary
Read the full report: https://www.reportlinker.com/p05089140
Over the past two decades, there has been increasing interest in Partial knee replacement (PKR) as a means of treating unicompartmental knee osteoarthritis. Indications have expanded while previous contraindications such as age, body mass index, patellofemoral osteoarthritis, and anterior cruciate ligament (ACL) deficiency have been lifted. Technological advancements in implant design, fixation methods, and surgical techniques have led to improved survival rates, better patient-reported outcomes, and reduced complications. Interest is growing in robotic-assisted techniques, which are poised to optimize results and reproducibility. Cementless designs also look promising, although given the anatomy of the knee condyles, this might introduce more risks to the overall procedure.
South American Partial knee replacement (PKR) market was estimated at $5+M in 2016 across the three markets (Argentina, Brazil, and Chile) covered in this report. By the end of the forecast period in 2023, it is estimated that the market will grow approximately at a Compound Annual Growth Rate (CAGR) of 7+%.Brazil represented the largest portion of the market in 2016, representing more than 70% of the regional revenue, and is expected to maintain this dominance through the forecast period. Argentina is expected to be the fastest-growing market through 2023.
As countries’ economy grows, the volume of partial knee replacement procedures is expected to increase further, primarily due to the demographics of an aging population and a trend towards early surgical intervention in younger patients. In the forecast years, launching and marketing a comprehensive large joint portfolio with a clear value proposition will remain critical to companies’ success as they jockey for market position. A long-term foothold within the global knee market hinges on how well companies can align themselves with the primary market force buffeting healthcare today: the need to deliver top-notch, cost-effective care.
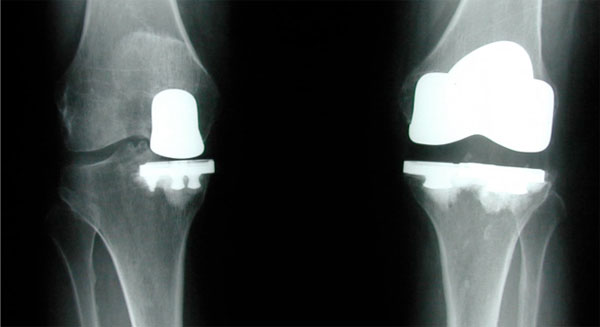
The incidences of osteoarthritis of the knee and other degenerative knee diseases are increasing simultaneously with the aging population. Partial knee replacement (PKR), or unicondylar knee arthroplasty (UKA), is an alternative to total knee arthroplasty (TKA) used to treat patients whose osteoarthritic condition is isolated to the medial or lateral tibio-femoral compartments. PKR is a less invasive procedure than TKA that replaces only the damaged part of the joint with a prosthesis, resulting in less blood loss and the preservation of healthy ligaments, cartilage, and bone in the knee. The decision to undergo a PKR instead of TKR is based on the disease stage, patient expectations, published results, and surgeon expertise.
Given the heightened competitive pressures and shrinking reimbursement, the average selling price for Partial knee replacement (PKR) implants is expected to decline. However, it is expected that the revenue from increased procedure volume will make up for this loss.
This report focuses on the market outlook for Partial knee replacement (PKR) devices in South America (Argentina, Brazil, and Chile). The analysis identifies the key unmet needs in the PKR market, discusses the major drivers and barriers of adoption, and provides an in-depth understanding of physicians’ perceptions and the future outlook for this market by segment and geography.
Key Questions Answered
– What is the current and future Partial knee replacement (PKR) market outlook in the developed and emerging markets? What trends are affecting the South American market?
– Which are the key, high growth markets that PKR manufacturers should expand into?
– What are the unmet needs with the current generation of Partial knee replacement (PKR) devices? How will emerging technologies fulfill these unmet needs?
– What are the challenges and complications that have hindered widespread adoption?
– With developing the next-generation of devices, what aspects of the technology are device manufacturers focused on optimizing? How will new entrants impact the Partial knee replacement (PKR) market?
Scope
– Overview of recent key industry events and analysis of their market impact.
– Annualized total market revenue, procedure trends, and market outlooks by segment and by region through 2023.
– Key topics covered include strategic competitor assessment, market characterization, identification of unmet needs, reimbursement considerations, evaluating market access in each region covered in the report, and implications of the emerging technologies in the market.
– Analysis of the current and future market competition in the global PKR market. Insightful review of industry drivers, barriers, and challenges.
Reasons to buy
The report will enable you to –
– Develop and design your in-licensing and out-licensing strategies through a review of marketed products and technologies.
– Develop business strategies by understanding the trends shaping and driving the video laparoscopes market.
– Drive revenues by understanding the key trends, innovative products and technologies, market segments, and companies likely to impact the video laparoscopes market in the future.
– Formulate effective sales and marketing strategies by understanding the competitive landscape and by analyzing the performance of various competitors.
– Identify emerging players with potentially strong product portfolios and create effective counter-strategies to gain a competitive advantage.
– Track device sales in country-specific video laparoscopes market from 2014-2023.
– Organize your sales and marketing efforts by identifying the market categories and segments that present maximum opportunities for consolidations, investments and strategic partnerships.
Read the full report: https://www.reportlinker.com/p05089140
About Reportlinker
ReportLinker is an award-winning market research solution. Reportlinker finds and organizes the latest industry data so you get all the market research you need – instantly, in one place.
__________________________
Contact Clare: clare@reportlinker.com
US: (339)-368-6001
Intl: +1 339-368-6001
SOURCE Reportlinker