AUDUBON, Pa., March 06, 2018 (GLOBE NEWSWIRE) — Globus Medical, Inc. (NYSE:GMED), a leading musculoskeletal solutions company, is pleased to announce its entry into the orthopedic trauma market. Globus Medical has become a market leader in spinal device innovation, offering differentiated products and disruptive technologies in a competitive spine market, making it one of the fastest growing companies in orthopedics. Integrating a well-known culture of speed to market and leveraging its robust product development engine, Globus Medical is poised to become a significant player in the orthopedic trauma market.
“Globus has demonstrated an unprecedented level of surgeon responsiveness resulting in the most comprehensive spinal product portfolio on the market. We aim to continue that tradition in orthopedic trauma by leveraging a dedicated product development team with a highly trained, direct sales force to bring our innovative and efficient systems along with the best support to our surgeon and hospital customers,” said Barclay Davis, Vice President, Orthopedic Trauma. “We feel we can capitalize on our strengths of innovation and speed to market to bring game changing technology in the trauma market.”
11 New Products FDA Cleared
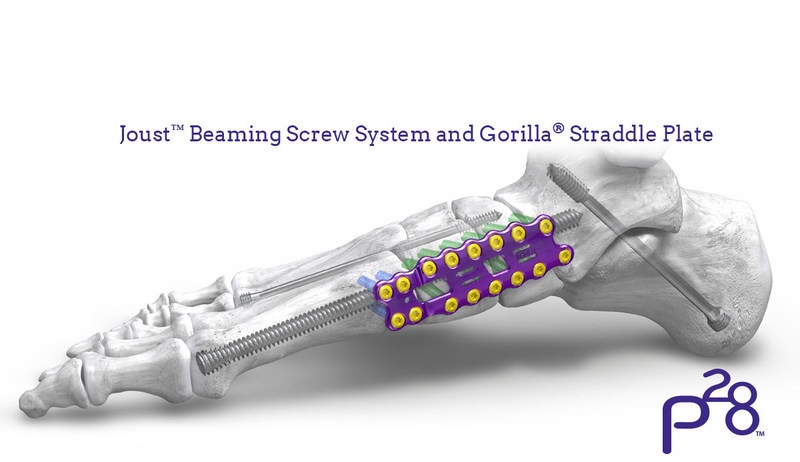
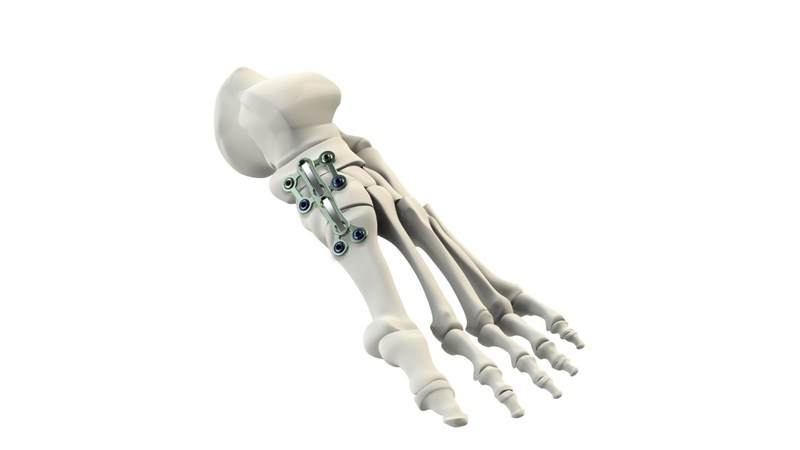
Globus Medical will display its growing suite of technology solutions for trauma products this week at the American Association of Orthopaedic Surgeons’ annual meeting in New Orleans. To date, Globus has received FDA 510(k) clearance for 11 products, covering the major segments of the orthopedic trauma market – fracture plates, compression screws, cannulated screws, intramedullary nails, and external fixation. This product portfolio is designed to treat a wide variety of fracture patterns and accommodates varying patient anatomies in the upper and lower extremities as well as hip and long bones. Each comprehensive system is optimized to streamline procedures and increase versatility, potentially reducing procedure time and expediting patient recovery.
Globus received FDA clearance for the following systems:
- Comprehensive ANTHEM Ankle Fracture System including advanced distal Fibula plates, Cannulated Screws and key Small Fragment Plating components
- ARBOR External Fixator System enabling fracture stabilization using one style clamp and one instrument
- AUTOBAHN Tibial Nailing System offering both infra-patellar and supra-patellar approaches and innovative locking options
- ANTHEM Small Fragment Plating System for general fractures with locking and non-locking implants and radiolucent instruments
- Comprehensive ANTHEM Distal Radius Fracture System for wrist fractures that includes innovative volar, dorsal, and lateral plates as well as a robust new bridge plate
- ANTHEM Proximal Humerus Plating System for shoulder fractures incorporating variable angle calcar screws and radiolucent instruments to simplify intra-op visualization and optimize implant placement
- AUTOBAHN Trochanteric Nailing System for efficient geriatric hip fracture reduction and fixation
- Comprehensive CAPTIVATE Compression Screw System for general fracture care including headless screws and an innovative variable-length family of screws for tough-to-reduce fractures or when bone purchase is limited
- Comprehensive AUTOBAHN Femoral Nailing System with Recon screw options in both Piriformis and Troch-entry style nails
- ANTHEM Proximal Tibia Plating System with innovative triple kick-stand screw fixation, integrated polyaxial rafting screws, and a novel radiolucent aiming arm for percutaneous fixation
- ANTHEM Clavicle Plating System with innovative plate contours optimized for clinically-based fracture zones
“It has been impressive watching Globus move from design to development to deployment of their comprehensive trauma solutions line,” said Dr. Andrew N. Pollak, Chief of Orthopaedics at the University of Maryland Medical System. “I have been very impressed with the ability of Globus’ engineers to translate surgeon ideas and concepts into functional and easy to use implants for a wide variety of fracture patterns.”
Discover Globus Medical’s orthopedic trauma product line at the American Association of Orthopaedic Surgeons’ annual meeting in New Orleans, March 6- 11th in Booth 4371 or visit www.globusmedical.com/trauma.
About Globus Medical, Inc.
Globus Medical, Inc. is a leading musculoskeletal solutions company based in Audubon, PA. The company was founded in 2003 by an experienced team of professionals with a shared vision to create products that enable surgeons to promote healing in patients with musculoskeletal disorders. Additional information can be accessed at http://www.globusmedical.com.
Safe Harbor Statements
All statements included in this press release other than statements of historical fact are forward-looking statements and may be identified by their use of words such as “believe,” “may,” “might,” “could,” “will,” “aim,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” “plan” and other similar terms. These forward-looking statements are based on our current assumptions, expectations and estimates of future events and trends. Forward-looking statements are only predictions and are subject to many risks, uncertainties and other factors that may affect our businesses and operations and could cause actual results to differ materially from those predicted. These risks and uncertainties include, but are not limited to, factors affecting our quarterly results, our ability to manage our growth, our ability to sustain our profitability, demand for our products, our ability to compete successfully (including without limitation our ability to convince surgeons to use our products and our ability to attract and retain sales and other personnel), our ability to rapidly develop and introduce new products, our ability to develop and execute on successful business strategies, our ability to comply with changing laws and regulations that are applicable to our businesses, our ability to safeguard our intellectual property, our success in defending legal proceedings brought against us, trends in the medical device industry, general economic conditions, and other risks. For a discussion of these and other risks, uncertainties and other factors that could affect our results, you should refer to the disclosure contained in our most recent annual report on Form 10-K filed with the Securities and Exchange Commission, including the sections labeled “Risk Factors” and “Cautionary Note Concerning Forward-Looking Statements,” and in our Forms 10-Q, Forms 8-K and other filings with the Securities and Exchange Commission. These documents are available at www.sec.gov. Moreover, we operate in an evolving environment. New risk factors and uncertainties emerge from time to time and it is not possible for us to predict all risk factors and uncertainties, nor can we assess the impact of all factors on its business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. Given these risks and uncertainties, readers are cautioned not to place undue reliance on any forward-looking statements. Forward-looking statements contained in this press release speak only as of the date of this press release. We undertake no obligation to update any forward-looking statements as a result of new information, events or circumstances or other factors arising or coming to our attention after the date hereof.
Contact:
Brian Kearns
Vice President, Business Development
Phone: (610) 930-1800
Email: investors@globusmedical.com
www.globusmedical.com