NESS ZIONA, Israel, March 30, 2017 /PRNewswire
CollPlant (TASE: CLPT), a regenerative medicine company utilizing its proprietary plant-based rhCollagen technology for tissue repair products (recombinant human, “rhCollagen”), today provided a commercial and operational update in its annual reports.
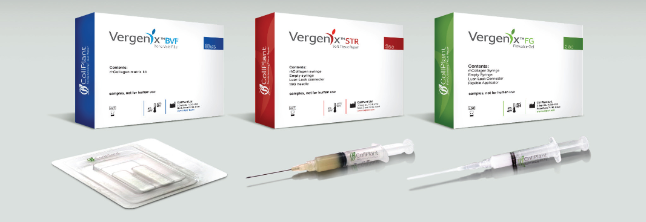
Most recently, in November 2016, the Company announced the signing of an exclusive distribution agreement with Arthrex GMBH to distribute Vergenix™STR, CollPlant’s CE Mark-approved treatment for tendinopathy, in Europe, and has supplied Arthrex with first orders. Arthrex, an affiliate of Arthrex Inc., based in Naples, FL, is a leading orthopedics company and is widely considered to be the world leader in sports medicine. Additionally, since mid-2016, CollPlant signed a number of marketing agreements for Vergenix™FG, its wound care product, covering Italy, Switzerland and Turkey. Commercial treatments have been completed over several months in Switzerland and Italy, and physicians’ feedback regarding patient results have been very positive.
CollPlant has also reported extensively for the first time on the development of its collagen-based biological ink, bioInk, for use in 3D printing of organs and tissues. Specifically, the Company is developing rhCollagen-based bioInk formulations for indications including skin, orthopedics, ophthalmology, heart and lung. The Company’s goal for the coming year includes working with a strategic international partner to further develop a specific indication of this product.
CollPlant’s Highlights for 2016 and the start of 2017
- Vergenix™FG – a wound filler based on the Company’s rhCollagen technology, for treatment of acute and chronic wounds: The product received CE Mark approval and distribution agreements have been signed in Italy, Switzerland and Turkey, including first orders, a portion of which were supplied in 2016. The initial target market for Vergenix™FG is estimated at $500 million annually. The Company has received positive feedback from physicians who have treated patients with the product in Europe.
- Vergenix™STR – a gel based on the Company’s rhCollagen technology, indicated for the treatment of tendinopathy: The product was granted CE Mark approval following successful clinical trials, and an exclusive distribution agreement was signed with Arthrex covering Europe, India and Africa. To date, CollPlant has reported on two orders supplied to Arthrex, and the product is currently in the initial stages of penetrating the European market. First treatments have commenced in Europe. The target market for Vergenix™STR is estimated at about three million procedures per annum worldwide, with a value of $2 billion.
- BioInk for 3D printing of organs and tissues – the Company is developing a biological ink (bioInk) based on its rhCollagen technology, intended for use with 3D printers, to print tissues and organs. In parallel, the Company is assessing potential joint ventures with international companies to further develop various applications such as skin, bones, ophthalmology, heart and lungs. The Company’s objective for this year is the development of a specific indication in order to achieve the creation of multilayer tissue combined with stem cells and other cells. The segment of 3D printing addresses the entire global medical market.
About CollPlant
CollPlant is a regenerative medicine company leveraging its proprietary, plant-based recombinant human collagen (rhCollagen) technology for the development and commercialization of tissue repair products, initially for the orthobiologics and advanced wound care markets. The Company’s cutting-edge technology is designed to generate and process proprietary rhCollagen, among other patent-protected recombinant proteins. Given that CollPlant’s rhCollagen is identical to the type I collagen produced by the human body, it offers significant advantages compared to currently marketed tissue-derived collagen, including improved biofunctionality, superior homogeneity and reduced risk of immune response. The Company’s broad development pipeline includes biomaterials indicated for orthopedics and advanced wound healing. Lead products include: Vergenix™STR (Soft Tissue Repair Matrix), for the treatment of tendinopathy; and Vergenix™FG (Flowable Gel) wound filler, for treatment of acute and chronic wounds. CollPlant’s business strategy includes proprietary development and manufacture of tissue repair products and their commercialization and distribution, together with leading third parties, alongside alliances with leading companies for joint development, manufacture and marketing of additional products.
For more information about CollPlant, visit http://www.collplant.com
Contact at CollPlant:
Eran Rotem
Chief Financial Officer
Tel: +972-73-2325600/612
Email: Eran@collplant.com
Contact at Rx Communications Group, LLC
Paula Schwartz (for US Investors)
Senior Vice President
Tel: +1-917-322-2216
Email: pschwartz@rxir.com
SOURCE CollPlan