TOULOUSE, France–(BUSINESS WIRE)–Regulatory News:
VEXIM (Paris:ALVXM) (FR0011072602 – ALVXM), a medical device company specializing in the minimally invasive treatment of vertebral fractures, today announces its consolidated Q1 sales results as of March 31st, 2017.
« VEXIM sales performance in Q1 is in line with our expectations. VEXIM continues to gain market share in Europe and Internationally with a market growing around 5%. Our German business is also progressing and we remain confident to reach at least €5 million of sales there and rank among the top 3 market leaders. We maintain our guidance of 30% to 35% growth of sales and aim at reaching profitability for the full year 2017 », said Vincent Gardès, CEO of VEXIM.
Continued growth in the first quarter 2017
Revenue (€ in million – IFRS, as of March, 31st)
|
Quarterly sales
|
| Q1 2017 |
|
Q1 2016 |
|
Variation (%) |
| 4.703 |
|
3.954 |
|
+19% |
€4.7 million in sales, VEXIM continues to expand in all geographies
In Europe, Vexim’s business continues to grow and the SpineJack® is becoming progressively the standard of care for vertebral fractures. In France, its domestic market, Vexim continues to grow and increase its market shares. In Germany, the biggest market in Europe (worth €70 million per year), Vexim increased the size of its salesforce to meet the growing demand of the market, for its products. With the addition of Masterflow Plus, Vexim’s secondary product specifically designed for the German market and to address a large potential of patients suffering from osteoporotic compression fractures, Vexim also gained access to Clinicpartners, Agaplesion, AGKAMed and Paul Gerhard Diakonie, counting for 250+ clinics for Vexim to sell into. Vexim will furthermore initiate a clinical study in Germany where the Spinejack® will be more evidence based towards a wider range of indications to follow. In Germany, Vexim is then pursuing the objective of achieving €5.0 million of sales in 2017, and ranking among the top 3 market leaders in the country.
Italy will see Vexim grow to achieve a 20% market share position. With the approval by Sanitas in Spain, one of the largest insurance companies, Vexim expects to see a major step forward in its market expansion in 2017.
Outside of Europe, where sales in Q1 increased by 114% vs Q1 2016, Vexim continues to see growing interest in the SpineJack® technology. As previously announced, Vexim is in the process of launching Spinejack® products in Brazil, Australia and South Korea in the coming months.
Since its launch in 2011, the Spinejack® has now been implanted to more than 46 000 patients which is close to 22 000 surgeries.
Confirmation of 2017 guidance
First quarter 2017 revenues are in line with Vexim’s expectations. The group confirms his guidance for full year 2017 :
- Sales growth of +30% to +35% (organic)
- Positive operating and net income, throughout the full year
- Sales of €5M Sales in Germany and rank among top 3 market leaders
Financial reporting schedule:
2nd quarter sales: July 11th, 20171
About VEXIM, the innovative back microsurgery specialist
Based in Balma, near Toulouse (France), VEXIM is a medical device company created in February 2006. The Company has specialized in the creation and marketing of minimally invasive solutions for treating traumatic spinal pathologies. Benefitting from the financial support of it longstanding shareholder, Truffle Capital2, and from OSEO public subsidies, VEXIM has designed and developed the SpineJack®, a unique implant capable of repairing a fractured vertebra and restoring the balance of the spinal column. The Company currently has 66 members on its staff. VEXIM has been listed on NYSE Alternext Paris since May 3rd 2012. For further information, please visit www.vexim.com
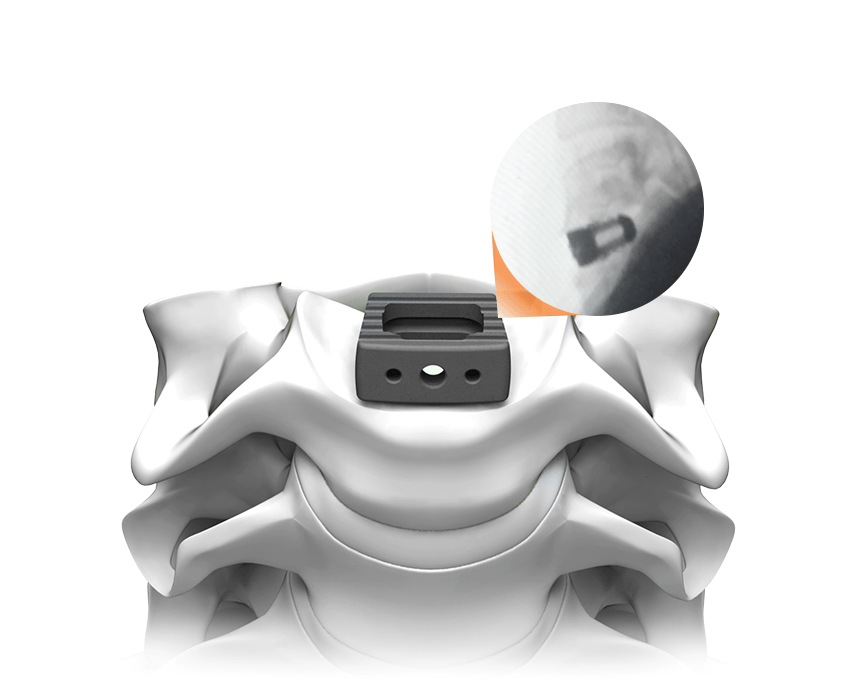
SpineJack®3, an innovative implant for treating Vertebral Compression Fractures
The SpineJack® is designed to restore a fractured vertebra to its original shape, restore the spinal column’s optimal anatomy and thus remove pain and enable the patient to recover their functional capabilities. Thanks to a specialized range of instruments, inserting the implants into the vertebra is carried out by minimally invasive surgery, guided by X-ray, in approximately 30 minutes, which is intended to enable the patient to be discharged shortly after surgery. The SpineJack® range consists of 3 titanium implants with 3 different diameters, thus covering 95% of vertebral compression fractures and all patient morphologies. SpineJack® technology benefits from the support of international scientific experts in the field of spine surgery and worldwide patent protection through to 2029.
1 Indicative date, subject to changes.
2 Founded in 2001 in Paris, Truffle Capital is a leading independent European private equity firm. It is dedicated to investing in and building technology leaders in the IT, life sciences and energy sectors. Truffle Capital manages €550m via FCPRs and FCPIs, the latter offering tax rebates (funds are blocked during 7 to 10 years). For further information, please visit www.truffle.fr and www.fcpi.fr.
3 This medical device is a regulated health product that, with regard to these regulations, bears the CE mark. Please refer to the Instructions for Use.
Nom : VEXIM
Code ISIN : FR0011072602
Code mnémonique : ALVXM