|
DUBLIN – May 25, 2017 – Medtronic plc (NYSE: MDT) today announced financial results for its fourth quarter and fiscal year 2017, which ended April 28, 2017. The company reported fourth quarter worldwide revenue of $7.916 billion, compared to the $7.567 billion reported in the fourth quarter of fiscal year 2016, an increase of 5 percent on both a reported and constant currency basis. Foreign currency translation had a negative $37 million impact on fourth quarter revenue. As reported, fourth quarter GAAP net income and diluted earnings per share (EPS) were $1.163 billion and $0.84, respectively. As detailed in the financial schedules included through the link at the end of this release, fourth quarter non-GAAP net income and diluted earnings per share (EPS) were $1.836 billion and $1.33, an increase of 2 percent and 5 percent, respectively. Fourth quarter U.S. revenue of $4.403 billion represented 56 percent of company revenue and increased 4 percent. Non-U.S. developed market revenue of $2.452 billion represented 31 percent of company revenue and increased 2 percent, or 4 percent on a constant currency basis. Emerging market revenue of $1.061 billion represented 13 percent of company revenue and increased 11 percent, or 10 percent on a constant currency basis. Medtronic’s fiscal year 2017 revenue of $29.710 billion increased 3 percent, or approximately 5 percent on a constant currency, constant week basis. Foreign currency translation had a negative $34 million impact on fiscal year 2017 revenue. The first quarter of fiscal year 2017 contained 13 weeks, one less week than the first quarter of fiscal year 2016. The extra week occurs every six years as a result of the company’s 52-53 week fiscal year calendar. While it is difficult to calculate an exact impact from the extra week, the company estimates that it resulted in an approximate $450 million benefit to revenue and $0.08 to $0.10 benefit to non-GAAP diluted earnings per share (EPS) in the first quarter of the prior fiscal year. As reported, fiscal year 2017 net earnings were $4.028 billion or $2.89 per diluted share. As detailed in the link at the end of this release, fiscal year 2017 non-GAAP earnings and diluted EPS were $6.395 billion and $4.60, representing increases of approximately 8 to 9 percent and approximately 11 to 12 percent, respectively, on a constant currency, constant week basis. “Our fourth quarter results were a strong finish to the fiscal year, with balanced, diversified growth across our groups and regions,” said Omar Ishrak, Medtronic chairman and chief executive officer. “Fiscal year 2017 was a solid year overall for Medtronic. We delivered record revenue, made progress in each of our growth strategies, executed on our Covidien cost synergy commitments, generated strong free cash flow growth, and deployed our capital in line with our stated priorities, balancing the return of cash to our shareholders together with disciplined reinvestment in our businesses.” Cardiac and Vascular Group
Minimally Invasive Therapies Group
Restorative Therapies Group
Diabetes Group
Guidance In fiscal year 2018, the company expects constant currency revenue growth to be in the range of 4 to 5 percent. While the impact of foreign currency is fluid, if current exchange rates remain similar for the remainder of the fiscal year, the company’s revenue would be positively affected by approximately $75 million to $175 million for the fiscal year, including an approximate negative $10 to negative $60 million impact in the first fiscal quarter. In fiscal year 2018, the company expects diluted non-GAAP EPS growth to be in the range of 9 to 10 percent on a constant currency basis. Assuming current exchange rates remain similar for the rest of the year, the company’s non-GAAP EPS would be negatively affected by approximately $0.05 to $0.10, including an approximate $0.03 to $0.05 impact in the first fiscal quarter. The company reiterated its long-term expectation of mid-single digit revenue growth and double digit EPS growth, both on a constant currency basis. In addition, the company noted that the fiscal year 2018 outlook and guidance does not include the impact of the previously announced divestiture of a portion of its Patient Monitoring and Recovery division to Cardinal Health, which the company continues to expect to close in the second fiscal quarter. The company intends to update its guidance upon close of the transaction. “We are creating distinct competitive advantages and capitalizing on the long-term trends in healthcare: namely, the desire to improve clinical outcomes; the growing demand for expanded access to care; and the optimization of cost and efficiency within healthcare systems. These trends, along with an aging population in most countries, produce secular growth tailwinds that we believe represent sustainable, long-term opportunities for Medtronic,” said Ishrak. “As we look forward, we have a number of catalysts that make us optimistic about our ability to deliver on our commitments and expand patient access around the world to our products and services. Our leadership team and employees continue to focus on driving excellence and impact in all that we do, and we look forward to the fiscal year ahead.” Webcast Information Financial Schedules About Medtronic FORWARD LOOKING STATEMENTS NON-GAAP FINANCIAL MEASURES Medtronic management believes that in order to properly understand its short-term and long-term financial trends, including period over period comparisons of the company’s operations, investors may find it useful to exclude the effect of certain charges or gains that contribute to or reduce earnings but that result from transactions or events that management believes may or may not recur with similar materiality or impact to operations in future periods (Non-GAAP Adjustments). Medtronic generally uses non-GAAP financial measures to facilitate management’s review of the operational performance of the company and as a basis for strategic planning. Non-GAAP financial measures should be considered supplemental to and not a substitute for financial information prepared in accordance with GAAP, and investors are cautioned that Medtronic may calculate non-GAAP financial measures in a way that is different from other companies. Management strongly encourages investors to review the company’s consolidated financial statements and publicly filed reports in their entirety. Reconciliations of the non-GAAP financial measures to the most directly comparable GAAP financial measures are included in the financial schedules accompanying this press release. Medtronic calculates forward-looking non-GAAP financial measures based on internal forecasts that omit certain amounts that would be included in GAAP financial measures. For instance, forward-looking revenue growth and EPS projections exclude the impact of foreign currency exchange fluctuations. Forward-looking non-GAAP EPS guidance also excludes other potential charges or gains that would be recorded as non-GAAP adjustments to earnings during the fiscal year, such as amortization of intangible assets and acquisition-related, certain tax and litigation, and restructuring charges or gains. Medtronic does not attempt to provide reconciliations of forward-looking non-GAAP EPS guidance to projected GAAP EPS guidance because the combined impact and timing of recognition of these potential charges or gains is inherently uncertain and difficult to predict and is unavailable without unreasonable efforts. In addition, we believe such reconciliations would imply a degree of precision and certainty that could be confusing to investors. Such items could have a substantial impact on GAAP measures of financial performance. View FY17 Fourth Quarter Financial Schedules & Non-GAAP Reconciliations Contacts: |
3 days / 6 sessions
Current Issues in Spine
May 25, 2017 – Medical Plastic News
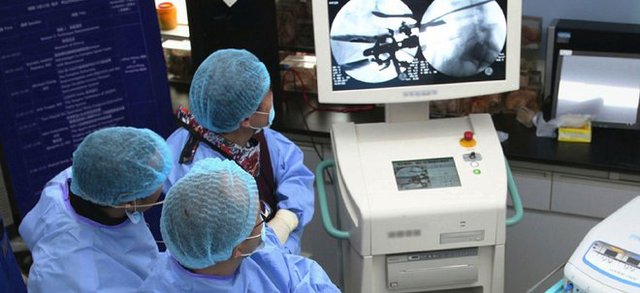
At the recent congress of the Chinese Association of Orthopaedic Surgeons (CAOS), Invibio Biomaterial Solutions of the UK, and China’s Double Medical Technology collaborated on an interbody spine surgery workshop to help expand knowledge of the implantation of Double Medical’s Direct Lateral Interbody Fusion (DLIF) spinal cages made with PEEK-Optima .
The biomaterial PEEK-Optima polymer by Invibio was introduced to medical device manufacturers in China after the approval by the China Federal Drug Administration (CFDA) in 2004.
Hosted in conjunction with the North American Spine Society (NASS), the CAOS workshop “Principles and Techniques of Complex Spine Surgery Workshop” took place on May 12, 2017, at the Zhongshan School of Medicine, Sun Yat-sen University, in Guangzhou. The event was the fifth joint NASS-CAOS workshop and delivered a full day of hands-on cadaver labs with over one hundred surgeons attending and multiple one-hour product demonstrations streamed live to the audience, including the demonstration of Double Medical and medical-grade PEEK innovator Invibio.
Double Medical, a large medical equipment group whose broad product range includes orthopaedic implants, dental implants, general surgical products, neurosurgical products and electronic medical devices, and Invibio have been working together since 2009. At this jointly planned NASS-CAOS event, the two companies demonstrated a Direct Lateral Interbody Fusion (DLIF) spinal cage made with PEEK-Optima and showed how the DLIF cage, which has already been used in lumbar surgeries, can provide a better overall therapeutic experience for patients.
May 25, 2017
BOCA RATON, Fla.–(BUSINESS WIRE)–SurGenTec®, a minimally invasive orthobiologics company, has announced that the United States Patent and Trademark Office has granted a new patent for its bone graft delivery technology.
The proprietary GRAFTGUN® delivery system enables surgeons to deliver the bone graft of their choice (synthetic, allograft, or autograft) in a minimally invasive fashion.
Traditionally, surgeons have used a metal funnel and tamp to place bone graft; these surgeons have sought a solution to overcome the difficulties and dangers with this technique. The GRAFTGUN provides a simple, safe and ergonomic solution to deliver bone graft in hard to reach places.
The GRAFTGUN kit includes a loading device and multiple tube sizes to support a variety of surgical procedures and patient sizes. The tubes have embedded radiopaque markers which enable the surgeon to visualize where their bone graft is being placed on fluoroscopy. The ratcheting technology provides the force necessary to extrude the majority of bone grafts on the market.
SurGenTec develops minimally invasive surgical technologies to help surgeons provide optimal care for their patients. SurGenTec believes the GRAFTGUN will be an asset to surgeons in both spine and orthopedic procedures.
The patent adds to SurGenTec’s portfolio of existing patents and patents pending.
SurGenTec also has several up-and-coming technologies that will be rolled out over the course of this year.
SurGenTec, a privately owned medical device company based out of Boca Raton, FL, plans to release the GRAFTGUN this summer.
Contacts
For SurGenTec
Andrew Shoup, 714-350-2546
Ashoup@SurGenTec.com
www.SurGenTec.com
QUEBEC CITY, QUEBEC, CANADA, May 24, 2017 /EINPresswire.com/ — Bodycad announced today that it has received product notification confirmation from the Belgian Federal Agency for Medicines and Health Products in Brussels, the legislative capital for the European Union, for its Bodycad Unicompartmental Knee System. This enables Bodycad to commercially launch this truly personalized orthopaedic restoration within the European Union in accordance with EU Medical Device Directive for custom devices. Bodycad is the first Canadian company to receive EU product notification confirmation for a joint reconstruction implant system.
Bodycad’s revolutionary Unicompartmental Knee System is designed to optimize personalized restoration of the patient’s unique anatomical features and kinematics. The system is based on proprietary 3D rendering of medical images of the patient’s anatomy. The restoration is delivered as a “procedure in a box” that completely revolutionizes the way orthopaedic implant and instrument applications are delivered and utilized in the operating room environment.
“The personalized restoration is created only after proper acquisition of data from the patient on an individualized level,” says Etienne Belzile, MD, orthopaedic surgeon and assistant professor at Laval University. “The benefit of personalized restoration is the possibility of a better fit to the individual, less trauma to the soft tissue, and potentially a faster recovery overall.”
Bodycad uses proprietary imaging algorithms to rapidly produce a precise 3D image of the patient’s knee. Its suite of Personalized Restoration Software enables a seamless integration of the image to implant process called the PREP (personalized restoration evaluation process). The efficient and rapid process is designed to increase patient satisfaction, improve manufacturing precision while improving economic quality metrics.
“Our proprietary software is based on 20 years of research in anthropometric data and is the first CAD/CAM software specifically developed for the personalization of orthopaedic implant and instrument design,” says Jean Robichaud, founder and CEO of Bodycad. “I am delighted to have European product notification confirmation to bring this important technological advancement to market. Our goal is to transform the way surgeons, patients and insurers think about the potential of mass customization to optimize patient care.”
About Bodycad
Bodycad is a Quebec City-based developer and manufacturer of personalized orthopaedics. Its personalized restorations offer patients a high level of conformity to their unique anatomy, with the potential for greater comfort, fit and durability that make the pursuit of orthopaedic perfection possible. Learn more at www.bodycad.com.
Andrew McLeod
Bodycad
1 418 527 1388
email us here
SUWANEE, GA–(Marketwired – May 25, 2017) – SANUWAVE Health, Inc. (OTCQB: SNWV) is pleased to announce that the company has appointed LITHOMED to act as distributors for SANUWAVE’s Orthopedic products in Taiwan.
Kevin Richardson II, CEO and Chairman, stated, “SANUWAVE has committed to deliver 3-5 new distribution partners in the second quarter and 7-10 by the end of the year. This is the first step in achieving or beating our target. We are also excited to be able to support our science advisor Dr. Chin-Jen Wang, Professor of Orthopedic Surgery at Chang Gung Memorial Hospital, and his newly founded Shockwave Institute in BANG DONG, Taiwan.”
André Mouton, V.P. International Sales and Relations of SANUWAVE, stated, “We are very pleased with this new relationship. LITHOMED has a wealth of experience within this specific indication and will be the right fit for our needs within the orthopedic market segment in Taiwan. Their access and relationships with Key Opinion Leaders (KOL’s) will prove to be of immense value for market development and revenue growth. Taiwan is the first country where we shall have a definitive split within our distribution model. We recognize that Orthopedics and Wound Care have a different customer base and the designated distributor needs to address that. We are expecting sales from this region to be well over $500,000 within the next three years, with a maximum value of $3 million. We have more than 200 of our devices in use in Europe and we are sure the Asia market and usage will progress accordingly. Taiwan will be our second territory within Asia,” concluded André Mouton.
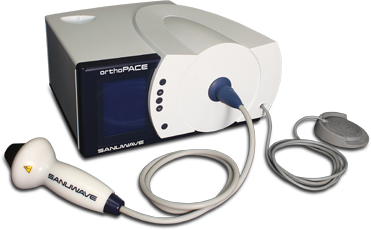
William Kao, Managing Director: “LITHOMED is very pleased to be the distributor for SANUWAVE’s orthopedic products in Taiwan. The orthoPACE® device, with its patented focused shock wave technology, is an important advancement in orthopedic and musculoskeletal care and offers a wide range of non-invasive treatment procedures for hospitals and doctor’s offices. I am certain it will be welcomed by Taiwanese orthopedists and their patients. These innovative products will fit perfectly within our portfolio and will be a welcomed addition to our current network of loyal customers and users. The orthoPACE device has been proven safe and effective for the treatment of chronic tendonitis and joint pain in the musculoskeletal environment. orthoPACE treatments have been especially effective in treating tendonitis and plantar fasciitis, which commonly require surgery. orthoPACE is designed to effectively treat bone conditions requiring osteogenesis, calcific joints, conditions causing painful joints, and chronic pain caused by musculoskeletal disorders. orthoPACE uses focused, shock wave treatments that are non-invasive which dramatically reduce the risk of infection. In many indications, orthoPACE has a proven success rate that is equal to or greater than that of surgery — usually with just one procedure and without the inherent risks, complications, or lengthy recovery time of invasive surgery. orthoPACE treatments require a minimal amount of treatment time. In conditions such as plantar fasciitis, patients can bear weight immediately and return to pre-treatment activity within a few days of the procedure,” concluded William Kao.
About SANUWAVE Health, Inc.
SANUWAVE Health, Inc. (OTCQB: SNWV) (www.sanuwave.com) is a shock wave technology company initially focused on the development and commercialization of patented noninvasive, biological response activating devices for the repair and regeneration of skin, musculoskeletal tissue and vascular structures. SANUWAVE’s portfolio of regenerative medicine products and product candidates activate biologic signaling and angiogenic responses, producing new vascularization and microcirculatory improvement, which helps restore the body’s normal healing processes and regeneration. SANUWAVE applies its patented PACE technology in wound healing, orthopedic/spine, plastic/cosmetic and cardiac conditions. Its lead product candidate for the global wound care market, dermaPACE®, is CE Marked throughout Europe and has device license approval for the treatment of the skin and subcutaneous soft tissue in Canada, Australia and New Zealand. In the U.S., dermaPACE is currently under the FDA’s de novo petition review process for the treatment of diabetic foot ulcers. SANUWAVE researches, designs, manufactures, markets and services its products worldwide, and believes it has demonstrated that its technology is safe and effective in stimulating healing in chronic conditions of the foot (plantar fasciitis) and the elbow (lateral epicondylitis) through its U.S. Class III PMA approved OssaTron® device, as well as stimulating bone and chronic tendonitis regeneration in the musculoskeletal environment through the utilization of its OssaTron, Evotron® and orthoPACE® devices in Europe, Asia and Asia/Pacific. In addition, there are license/partnership opportunities for SANUWAVE’s shock wave technology for non-medical uses, including energy, water, food and industrial markets.
About LITHOMED
Litho Med Trading Company has been established in 1994. The company specializes in distribution of medical equipment with a key focus on Shockwave Technology. It has been involved with clinical studies with OssaTron in 1998 and the company is closely working with the Key Opinion Leaders (KOL’s) within Shockwave technology within Taiwan since. The company is the sole distributor for Edaptms for UST since 2010. The orthoPACE technology and usage will be an added indication to complete the current product offering.
Litho Med Trading Co., Ltd.
TEL:06-2903269 FAX:06-2903297
3F-7, No.293 Sec 3, Dung-Men Road.
Tainan 701, Taiwan, R.O.C.
Forward-Looking Statements
This press release may contain “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, such as statements relating to financial results and plans for future business development activities, and are thus prospective. Forward-looking statements include all statements that are not statements of historical fact regarding intent, belief or current expectations of the Company, its directors or its officers. Investors are cautioned that any such forward-looking statements are not guarantees of future performance and involve risks and uncertainties, many of which are beyond the Company’s ability to control. Actual results may differ materially from those projected in the forward-looking statements. Among the key risks, assumptions and factors that may affect operating results, performance and financial condition are risks associated with the regulatory approval and marketing of the Company’s product candidates and products, unproven pre-clinical and clinical development activities, regulatory oversight, the Company’s ability to manage its capital resource issues, competition, and the other factors discussed in detail in the Company’s periodic filings with the Securities and Exchange Commission. The Company undertakes no obligation to update any forward-looking statement.
For additional information about the Company, visit www.sanuwave.com.
CONTACT INFORMATION
- Millennium Park Capital LLC
Christopher Wynne
312-724-7845
cwynne@mparkcm.comSANUWAVE Health, Inc.
Andre Mouton
Vice President International Sales and Relations
+1-615-823-9907 (Cell)
+1-678-569-0881(Fax)
Skype: andre.w.mouton
Andre.Mouton@sanuwave.com
TORONTO–(BUSINESS WIRE)–Antibe Therapeutics Inc. (“Antibe” or the “Company“) (TSXV: ATE) (OTCQX: ATBPF) a diversified biotechnology company announced today that it has filed a preliminary short form prospectus in connection with a proposed marketed offering of units of the Company (the “Units”) for minimum gross proceeds of $3,000,000 and maximum gross proceeds of $5,000,000 (the “Offering”). Each Unit will be comprised of one common share of the Company and one half of one common share purchase warrant (each full warrant, a “Warrant”). The Offering will be undertaken on a “best efforts” agency basis in the provinces of Ontario, British Columbia and Alberta pursuant to the Company’s preliminary short form prospectus dated May 24, 2017 (the “Preliminary Prospectus”), filed with securities regulators in Ontario, British Columbia and Alberta.
The number of Units to be distributed, the price of each Unit and the exercise price and term of each Warrant will be determined in the context of the market. Bloom Burton Securities Inc., Dominick Inc., and Echelon Wealth Partners Inc. are co-agents for the Offering (together, the “Agents”).
The Company has granted the Agents an over-allotment option (the “Over-Allotment Option”), exercisable in whole or in part, at the Agents’ sole discretion, at any time and from time to time for a period of 30 days following the final closing, to offer and sell on the same terms as the Offering up to such number of additional Units as is equal to 15% of the number of Units issued under the Offering.
In consideration for the services to be rendered by the Agents in connection with the Offering, the Agents will receive a fee consisting of cash and broker warrants.
If the minimum offering size is completed, the Company intends to use the net proceeds to: (i) complete its Phase 2 GI safety study for the Company’s lead drug, ATB-346 and (ii) commence metabolic studies for ATB-346. If the maximum offering size is completed, the Company intends to use the net proceeds to: (i) complete its Phase 2 GI safety study for ATB-346; (ii) commence its Phase 2 dose-ranging efficacy clinical study for ATB-346; (iii) fully fund the metabolism studies of ATB-346; and (iv) partially fund IND-enabling pre-clinical studies for its second pipeline drug, ATB-352. In addition to clinical development, the Company intends to use a portion of the net proceeds of the Offering for Citagenix product and business development, working capital and general corporate purposes. For additional detail regarding the use of proceeds, please refer to the Preliminary Prospectus.
The Offering is subject to a number of conditions, including, without limitation, receipt of all regulatory approvals, including the approval of the TSX Venture Exchange. There can be no assurance as to whether or when the Offering may be completed, or as to the actual size or terms of the Offering.
A copy of the Preliminary Prospectus, which was filed in each of the provinces of Ontario British Columbia and Alberta, contains important information relating to the Offering and the Units, and is available on SEDAR at www.sedar.com or by contacting Bloom Burton Securities Inc., at ecm@bloomburton.com. The Preliminary Prospectus is still subject to completion or amendment. There will not be any sale or any acceptance of an offer to buy the Units until a receipt for the final prospectus relating to the Offering has been issued.
This press release does not constitute an offer to sell or a solicitation of an offer to buy the Units in any jurisdiction, nor will there be any offer or sale of the Units in any jurisdiction in which such offer, solicitation or sale would be unlawful. The Units have not and will not be registered under the United States Securities Act of 1933, as amended (the “U.S. Securities Act”), or any U.S. state securities laws, and therefore will not be offered or sold within the United States except pursuant to exemptions from the registration requirements of the U.S. Securities Act and applicable state securities laws.
About Antibe Therapeutics Inc.
Antibe develops safer medicines for pain and inflammation. Antibe’s technology involves linking a hydrogen sulfide-releasing molecule to an existing drug to produce a patented, improved medicine. Antibe’s lead drug ATB-346 targets the global need for a safer drug for chronic pain and inflammation. ATB-352, the second drug in Antibe’s pipeline, targets the urgent global need for a safer, non-addictive analgesic for treating severe acute pain, while ATB-340 is a GI-safe derivative of aspirin.
Antibe’s subsidiary, Citagenix Inc. (“Citagenix”), is a leader in the sales and marketing of tissue regenerative products servicing the orthopedic and dental marketplaces. Since its inception in 1997, Citagenix has become an important source of knowledge and experience for bone regeneration in the Canadian medical device industry. Citagenix is active in 15 countries, operating in Canada through its direct sales teams, and internationally via a network of distributor partnerships.
Forward-Looking Information
This news release contains certain “forward-looking information” as such term is defined under applicable Canadian securities laws. All statements, other than statements of historical fact, that address activities, events or developments that the Company believes, expects or anticipates will or may occur in the future (including, without limitation, statements relating to the Offering generally, the terms thereof and the use or proceeds from the Offering) constitute forward-looking information. This forward-looking information reflects the current expectations or beliefs of the Company based on information currently available to the Company as well as certain assumptions including, without limitation, the ability of the Company to complete the Offering in a timely manner and on the terms and conditions described in the news release). Forward-looking information is subject to a number of significant risks and uncertainties and other factors that may cause the actual results of the Company to differ materially from those discussed in the forward-looking information, and even if such actual results are realized or substantially realized, there can be no assurance that they will have the expected consequences to, or effects on the Company. Factors that could cause actual results or events to differ materially from current expectations include, but are not limited to, the risk that the Company may not be able to raise the minimum amount of proceeds required to complete the Offering; the failure of the Company to effectively obtain the approval of the TSX Venture Exchange for the Offering; the inability of the Company to satisfy all conditions to the completion of the Offering and the risk of unforeseen delays in the completion of the Offering, if at all, whether as a result of market conditions or otherwise. Reference is also made to the risk factors disclosed under the heading “Risk factors” in the Company’s AIF for the year ended March 31, 2016 which has been filed on SEDAR and is available under the Company’s profile at www.sedar.com.
The TSX Venture Exchange has in no way passed upon the merits of the proposed Offering and has neither approved nor disapproved the contents of this press release. Neither the TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
Contacts
Antibe Therapeutics Inc.
Dan Legault, +1 416-473-4095
Chief Executive Officer
dan.legault@antibethera.com
MAHWAH, N.J., May 24, 2017 /PRNewswire/ — Stryker Orthopaedics announced today plans to activate in Fort Worth, TX for the DEAN & DELUCA Invitational. For the second consecutive year appearing at the tournament, Stryker will continue to engage with fans through its onsite activation, The Mobility Zone – a destination designed to educate golf fans on the importance of joint health through various engaging activities. New for 2017, on Friday, Army Veteran Robert Baker, Stryker’s Vice President of Operations, will present one well-deserving military hero with a service dog in a special K9s For Warriors ceremony – marking the company’s third donation this year.
As an extension of a successful relationship that began last year, throughout the PGA TOUR® and PGA TOUR Champions 2017 season, Stryker will continue to give a new leash on life to military heroes by sponsoring service canines and empowering veterans to return to civilian life with dignity and independence. Tournament goers can also show their support to veterans by purchasing the same hat that PGA TOUR professionals and longtime brand ambassadors, Fred Funk and Hal Sutton wear on TOUR at the newly renovated Mobility Zone – Stryker’s premiere “joint health” destination. With each hat purchase, Stryker will make a donation to the K9s For Warriors organization.
This year marks the 71st anniversary of the tournament being held at Colonial Country Club – making it the longest running PGA TOUR event at the same venue. While on site, Stryker will showcase its commitment to educate Colonial’s dedicated tournament goers about the importance of leading an active lifestyle and having healthy joints. Inside The Mobility Zone, fans can partake in the new Stryker Challenge – a hands-on experience featuring Art H. Ritis, a life-size model that aims to provide tournament goers with a basic understanding of joint replacement surgery and Stryker’s products. In addition, fans who stop by The Mobility Zone will be able to enter for a chance to win a trip for two to Atlanta, GA for the TOUR Championship® and walk inside the ropes as an honorary observer – a true VIP experience.1,2
“We are proud to continue to serve as a resource for fans onsite to learn about the importance of joint health, while also paying tribute to our brave service men and women,” said Bill Huffnagle, President, Stryker’s Joint Replacement Division. “Tournament goers can visit The Mobility Zone to support our military by purchasing a Stryker hat and learn more about joint health through fun activities and challenges.”
In an effort to reach fans both on and off the golf course, the company recently embarked on a cross- country road trip with brand ambassadors, NFL Hall of Famer Jerome Bettis and PGA TOUR Champions player and GetAroundKnee® patient, Fred Funk. The two have teamed up alongside Stryker to create a video series, demonstrating various ways to stay healthy and keep your joints moving. Earlier this month, Bettis and Funk stopped by Forth Worth to film the second episode of the video series where they went to the local batting cages to hit a few balls, and made a pit stop at the nation’s largest honky-tonk to learn how to line dance. To follow the “Road Trip to a Healthier Lifestyle” series and learn more about Stryker, visit StrykerChallenge.com.
- Healthcare Professionals (HCPs) are not eligible to enter the Stryker Challenge Sweepstakes or participate in the any of these promotions. HCPs are defined as those individuals or entities involved in the provision of health care services and/or items to patients, which purchase, lease, recommend, use, arrange for the purchase or lease of, or prescribe Stryker’s products.
- No purchase necessary to enter or win Sweepstakes. Void where prohibited by law. For official rules visit StrykerChallenge.com. Open to legal residents of the US & US Territories, 21+ as of date of entry. Sweepstakes begins at 12:01 am ET on 1/11/17 and ends at 11:59 pm ET on 8/27/17. Sponsored by Stryker.
About Stryker
Stryker is one of the world’s leading medical technology companies and, together with our customers, we are driven to make healthcare better. The Company offers a diverse array of innovative products and services in Orthopaedics, Medical and Surgical, and Neurotechnology and Spine that help improve patient and hospital outcomes. Stryker is active in over 100 countries around the world.
About PGA TOUR
The PGA TOUR is the leading global platform in professional golf, showcasing the highest expression of excellence, both on and off the course. The PGA TOUR’s mission is to entertain and inspire its fans, deliver substantial value to its partners, create outlets for volunteers to give back, generate significant charitable and economic impact in the communities in which it plays, grow and protect the game of golf and provide financial opportunities for TOUR players.
The PGA TOUR co-sanctions more than 130 tournaments on the PGA TOUR, PGA TOUR Champions, Web.com Tour, PGA TOUR Latinoamérica, Mackenzie Tour-PGA TOUR Canada and PGA TOUR China. Its members represent the world’s best players, hailing from 24 countries (86 members are from outside the United States). Worldwide, PGA TOUR tournaments are broadcast to more than 1.1 billion households in 227 countries and territories in 23 languages. Virtually all tournaments are organized as non-profit organizations in order to maximize charitable giving. In 2016, tournaments across all Tours generated a record of more than $166 million for local and national charitable organizations, bringing the all-time total to $2.46 billion.
The PGA TOUR’s web site is PGATOUR.COM, the No. 1 site in golf, and the organization is headquartered in Ponte Vedra Beach, Fla.
SOURCE Stryker
May 24, 2017
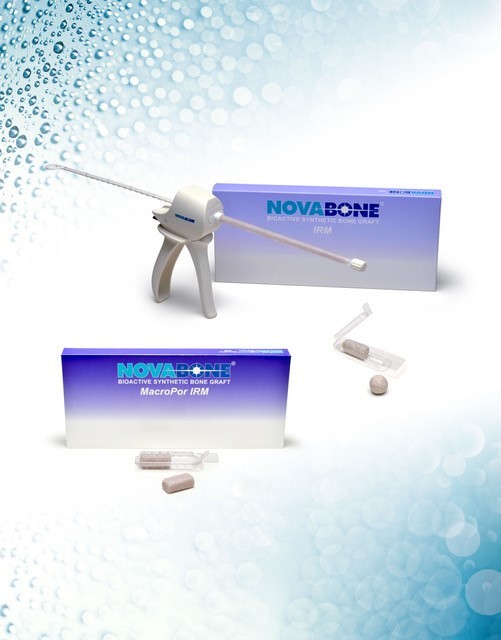
JACKSONVILLE, Fla.–(BUSINESS WIRE)–NovaBone Products, a leading biologics medical device company, announces the addition of NovaBone IRM and NovaBone IRM MacroPOR to its portfolio of biologically active bone graft substitutes.
NovaBone IRM, and NovaBone IRM MacroPOR are the most advanced bioactive synthetic bone graft substitutes available to the orthopedic community today. Both formulations optimize osteogenesis, uniquely signal and stimulate osteoblastic activity, and offer angiogenic potential. By design, both formulations are highly irrigation resistant with excellent cohesion and handling properties.
“NovaBone Products’ base Bioactive Glass technology has proven to out-perform traditional calcium sulfate based synthetic products and demineralized bone matrix products,” said Dennis McBride, Vice President of Sales and Marketing. “We are now advancing our science further by providing formulations with carriers that have a synergistic effect in bone formation and retain molded shapes following implantation and irrigation.”
Novabone offers the broadest portfolio of bioactive synthetic bone graft substitutes in the world, with a robust pipeline of products in development designed to expand the regenerative biologics products the company offers. Presently, products offered include minimally invasive (MIS) delivery devices, strip formulations, putty materials that are hydrated using bone marrow aspirate, and putty formulations that are ready-to-use out of the package.
“We are proud to offer products that allow a surgeon to look to one company when addressing bone grafting needs,” concluded McBride.
NovaBone Products, a privately held company based in Florida, USA since 2002 and a recognized industry leader in bioactive glass design and manufacturing, developed the first bioactive synthetic bone graft offered to the orthopaedic community.
Contacts
NovaBone Products
Arthur Wotiz, President
904-651-0607
awotiz@novabone.com
DALLAS, TEXAS, USA, May 24, 2017 /EINPresswire.com/ — Gramercy Extremity Orthopedics (GEO) is pleased to announce the first surgery using the GEO™ Bone Screw and GEO CART™ systems. The first surgery was performed by Dr. Peter A. Blume, D.P.M., F.A.C.F.A.S. at Shoreline Surgery Center LLC, Guilford, CT, with outstanding results. As the first offering in the company’s product line, this marks a major milestone for Gramercy Extremity Orthopedics™.
Dr. Blume remarked on the advantages of the GEO™ Bone Screw and GEO CART™ Systems, “I was extremely impressed and pleased with the outcome when utilizing the GEO™ Bone Screw and GEO CART™ systems. The ease in which the system operated was noted by the entire Surgical Center staff and felt that the systems created many efficiencies that are lacking in today’s operating environment. The surgical back table was free of clutter and the implant and instruments were truly best of class. The GEO CART™ finally addresses all the pitfalls when operating with orthopedic hardware, whether it be at the Surgery Center or in the hospital setting.”
The GEO CART™ proprietary point-of-sale system reduces delays in surgery, decreases sterility risks to the patient, eliminates billing mistakes and hand written forms, automatically generates the Implant Usage Form and reduces facility operating expenses.
The GEO CART™ is a computerized mobile implant and instrumentation inventory system based on RFID technology. No bigger than the average medical cart, the GEO CART™ system can hold over 2,000+ items.
The GEO™ Bone Screw System offers a comprehensive array of low profile titanium screw lengths, diameters, thread lengths and fully threaded options. All GEO™ Bone Screws are double sterile packaged, self-drilling, self-tapping, reverse-cutting with variable length short and long threads and a hexalobe head to provide additional stability and torque transfer with less potential for head stripping. All instruments within the GEO™ Bone Screw system are also double sterile packaged in single use kits to ensure a new and sterilized instrument is used every surgery.
“We are all very pleased with the results from today’s surgery. Not only did the implant and instruments all work superbly, the case was performed without issue and in an efficient manner,” says Michael P. Simpson, President and CEO of Gramercy Extremity Orthopedics™. “As for the GEO CART™, in my 16 years in orthopedic medical devices, I have never had the ability to see the transactions remotely from a case within minutes of the surgery happening. This is a significant breakthrough in our industry. I want to thank the GEO™ team for all of their hard work in getting this system to the marketplace”
GEO™ will be exhibiting at the AOFAS Scientific Conference, Seattle, July 12-14, 2017. Booth #610.
About Gramercy Extremity Orthopedics™:
GEO™ was formed from the idea that there could exist a more cost-effective, user-friendly way to supply Orthopedic Medical Implants in today’s healthcare environment. This is accomplished through the use of RFID technology, a groundbreaking Point-of-Sale delivery system, and GEO™ designed best in class sterilized single-use orthopedic implants and instruments. GEO™ is the only solution that provides a significant opportunity to lower real operating costs by creating efficiencies and controls throughout the delivery and consumption of orthopedic implants.
GRAMERCY EXTREMITY ORTHOPEDICS™, GEO™, and GEO CART™ are proprietary trademarks of Gramercy Extremity Orthopedics™. All rights reserved.
Michael Nugent
Gramercy Extremity Orthopedics™
855-436-2278 ext 103
email us here
SOMERVILLE, N.J., May 24, 2017 /PRNewswire/ — Ethicon* announced the results of two economic analyses demonstrating that the DERMABOND® PRINEO® Skin Closure system, a product that combines a topical skin adhesive with a self-adhering patch, is associated with improved patient outcomes and lower healthcare costs for hospitals. Both studies are being presented at the International Society for Pharmacoeconomics and Outcomes Research’s 22nd Annual International Meeting this week.
The first study, Comparison of Economic and Clinical Outcomes between the DERMABOND® PRINEO® Skin Closure System and Skin Staples in Patients Undergoing Knee Replacement in Real World Clinical Practice,1 found that the DERMABOND PRINEO System is associated with improved outcomes for patients receiving total knee arthroplasty (TKA) when compared to skin staples. The findings are based on a retrospective analysis of 1,942 TKA procedures using the Premier Perspective® Hospital Database. Specifically, the DERMABOND PRINEO System was associated with a statistically significant reduction in readmission rates at 30, 60 & 90 days, a reduction in length of hospital stay and a lower probability of being discharged to a skilled nursing facility (SNF). The analysis showed that the 30-day readmission rate for the DERMABOND PRINEO System group was 1.8%, compared to 4.4% for the skin staple group. The costs of 30 day readmissions for TKA have been estimated to be $12,839.2 The DERMABOND PRINEO System was also associated with a 12% reduction in length of hospital stay and a 31% reduction in discharge to a SNF or other non-home setting, which could lead to savings in the acute and post-acute setting.
The second study, A U.S. Hospital Budget Impact Analysis of a Skin Closure System Compared with Standard of Care in Hip and Knee Arthroplasty, estimates the 90-day cost impact of the DERMABOND PRINEO System compared to other wound closure methods for hip and knee arthroplasty from a U.S. provider perspective.3 The analysis showed that the use of the DERMABOND PRINEO System DERMABOND PRINEO System in hip and knee arthroplasty may achieve cost savings that could translate into an annual hospital budgetary savings ranging from $28,349 to $39,809 when assuming 500 arthroplasties.3 The predicted cost savings was driven by reductions in dressing materials and post-operative healthcare visits when the DERMABOND PRINEO System is utilized.
“Ethicon is committed to bringing to market innovative products that are designed to provide improved outcomes for patients and enable our customers to provide the best care for their patients,” says Nefertiti Greene, Vice President, Global Wound Closure and Repair Platform Leader at Ethicon. “The DERMABOND PRINEO System has been shown by this research to be an excellent approach to wound closure.”
Reducing the length of a patient’s hospital stay can lead to reduced costs and may also lower the chances of contracting an infection.4In addition, reducing readmissions can prevent hospitals from being penalized for readmission rates above the national average for TKA.5 The reductions in health care resource utilization demonstrated in these studies are important for hospital systems and health care professionals particularly those who are part of or are considering episode-based payments where both acute and post-acute costs are combined into one payment.
Several benefits associated with the DERMABOND PRINEO System may explain the findings. The product provides significantly greater skin holding strength than skin staples and subcuticular suture,6+ and acts as a barrier to microbial penetration against organisms commonly associated with surgical site infection.7 The DERMABOND PRINEO System requires no postsurgical dressings which may mean easier self-care and greater self-confidence for patients.7 Also, if directed by their healthcare professional, patients can shower immediately after surgery7 which could lead to a higher level of patient satisfaction.
About Ethicon
From creating the first sutures to revolutionizing surgery with minimally invasive procedures, Ethicon, part of the Johnson & Johnson Medical Devices Companies, has made significant contributions to surgery for more than 60 years. Our continuing dedication to Shape the Future of Surgery is built on our commitment to help address the world’s most pressing health care issues, and improve and save more lives. Through Ethicon’s surgical technologies and solutions including sutures, staplers, energy devices, trocars and hemostats and our commitment to treating serious medical conditions like obesity and cancer worldwide, we deliver innovation to make a life-changing impact. Learn more at www.ethicon.com, and follow us on Twitter @Ethicon.
*Ethicon represents the products and services of Ethicon, Inc., Ethicon Endo-Surgery, LLC and certain of their affiliates Ethicon, Inc. is the legal manufacturer of the DERMABOND® PRINEO® Skin Closure system. All other trademarks are the property of their respective owners.
+In an ex vivo study, more load in N was required to create a 3-mm gap between skin edges approximated with DERMABOND PRINEO System (22 cm) than with subcuticular 4-0 MONOCRYL® (poliglecaprone 25) Suture or PROXIMATE® Ethicon Endo-Surgery skin staples (P<.001).
073076-170517
1 Johnston S, Sutton N. Comparison of Economic and Clinical Outcomes between the Dermabond® Prineo® Skin Closure System and Skin Staples in Patients Undergoing Knee Replacement in Real World Clinical Practice. Poster Presented at: ISPOR 22nd Annual International Meeting; May 20-24, 2017; Boston, MA.
2 HCUP Statistical Briefs website. Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sbtopic.jsp. Accessed May 17, 2017
3 Sadik, K, Flener J, Gargiulo J, Graves M, Nunley, R Post, Z, Wurzelbacher, Sutton, N, Hogan, Hollman, S, Ferko, N. A U.S. Hospital Budget Impact Analysis of a Skin Closure System Compared with Standard of Care in Hip and Knee Arthroplasty. Poster Presented at: ISPOR 22nd Annual International Meeting; May 20-24, 2017; Boston, MA.
4 Hassan M, Tuckman HP, Patrick RH, Kountz DS, Kohn JL. Hospital length of stay and probability of acquiring infection. International Journal of Pharmaceutical and Healthcare Marketing. 2010;4(4):324-338.
5 Centers for Medicare & Medicaid Services Readmissions Reduction Program (HRRP) https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program.html. Accessed May 17, 2017.
6 Data on file, Ethicon, Inc.: Kumar A. AST-2012- 0290. Completion Report: Study to compare the tissue holding strength of PRINEO Skin Closure System with conventional wound closure techniques. 2012.
7 Data on file. Ethicon, Inc. Dermabond Prineo System Master Claims Matrix.
SOURCE Ethicon