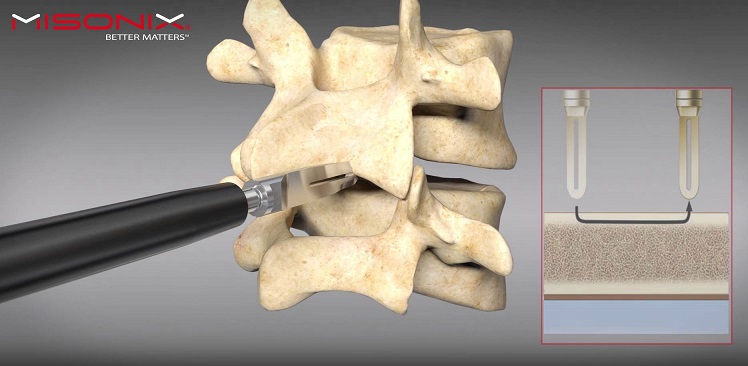
FARMINGDALE, N.Y., Oct. 25, 2017 (GLOBE NEWSWIRE) — Misonix, Inc. (NASDAQ:MSON), a provider of minimally invasive therapeutic ultrasonic medical devices that enhance clinical outcomes, today cited data that demonstrated the use of BoneScalpel can produce up to a 40% cost reduction in biologic grafting material spend in spinal fusion procedures.
The data was presented today by Dr. Wade Jensen of the Center for Neurosciences, Orthopaedics & Spine (“CNOS”), at the 32nd Annual Meeting of the North American Spine Society (“NASS”) in Orlando, Florida. The data showed an average cost savings of $1,066 in bone graft material when the Misonix BoneScalpel was employed compared to the use of standard power drill instruments. The Misonix BoneScalpel ultrasonically cuts bone using a 1mm thick blunt blade. The result is a clean cut that preserves healthy bone which can be used for grafting, and spares the surrounding soft tissue. The study is based on a retrospective analysis including 144 cases, 44 of which were done using the Misonix BoneScalpel. The remaining 100 cases were done with a 3mm rotating matchstick drill bit (“burr”) currently in broad use in surgical suites throughout the U.S. An estimated $1.9 billion is spent annually on bone graft substitutes.
Stavros Vizirgianakis, President and Chief Executive Officer of Misonix, said, “The data presented today are a strong validation of the efficacy of the BoneScalpel in producing significant cost savings in the surgical suite. This data demonstrates the value of using ultrasonic bone removal over standard electric power instruments both in the precision of the bone removal and the reduction in overall bone destruction. The ability to ultrasonically remove bone in a manner that enhances the ability for successful bone grafting while minimizing the need for biological grafting material represents an important step forward for patients, surgeons and medical institutions. We believe that the BoneScalpel can become the standard of care for precision bone cutting while sparing soft tissue and delivering significant cost savings to the healthcare process.”
Misonix will be exhibiting at NASS 2017 at booth #765 from Wednesday, October 25, 2017 through Saturday, October 28, 2017.
About NASS
The North American Spine Society (“NASS”) is a global multidisciplinary medical society that utilizes education, research and advocacy to foster the highest quality, ethical, value- and evidence-based spine care for patients.
About Misonix
Misonix, Inc. designs, develops, manufactures and markets therapeutic ultrasonic medical devices. Misonix’s therapeutic ultrasonic platform is the basis for several innovative medical technologies. Addressing a combined market estimated to be in excess of $1.5 billion annually; Misonix’s proprietary ultrasonic medical devices are used in spine surgery, neurosurgery, orthopedic surgery, wound debridement, cosmetic surgery, laparoscopic surgery, and other surgical and medical applications. Additional information is available on the Company’s Web site at www.misonix.com.
Safe Harbor Statement
With the exception of historical information contained in this press release, content herein may contain “forward looking statements” that are made pursuant to the Safe Harbor Provisions of the Private Securities Litigation Reform Act of 1995. These statements are based on management’s current expectations and are subject to uncertainty and changes in circumstances. Investors are cautioned that forward-looking statements involve risks and uncertainties that could cause actual results to differ materially from the statements made. These factors include general economic conditions, delays and risks associated with the performance of contracts, risks associated with international sales and currency fluctuations, uncertainties as a result of research and development, acceptable results from clinical studies, including publication of results and patient/procedure data with varying levels of statistical relevancy, risks involved in introducing and marketing new products, potential acquisitions, consumer and industry acceptance, litigation and/or court proceedings, including the timing and monetary requirements of such activities, the timing of finding strategic partners and implementing such relationships, regulatory risks including approval of pending and/or contemplated 510(k) filings, the ability to achieve and maintain profitability in the Company’s business lines, the impact of the pending investigation by the Department of Justice and Securities Exchange Commission, and other factors discussed in the Company’s Annual Report on Form 10-K, subsequent Quarterly Reports on Form 10-Q and Current Reports on Form 8-K. The Company disclaims any obligation to update its forward-looking relationships.
Corporate Contact
Misonix Contact:
Joe Dwyer
631-694-9555
invest@misonix.com
Investor Contact
Joe Diaz
Lytham Partners
602-889-9700
info@misonix.com