SOMERVILLE, N.J., Aug. 24, 2016 /PRNewswire-USNewswire/ — Ethicon, Inc., part of the Johnson & Johnson Medical Devices Companies, announces a strategic collaboration with Touch Surgery to help improve patient outcomes by delivering simulated surgical training based on the safe and efficacious use of Ethicon products in a free mobile app that can reach medical professionals in even remote regions of the world.
Ethicon’s more than 60-year history in educating surgeons and deep expertise with surgical products, combined with Touch Surgery’s technology platform and unique 3D simulations, will provide digital content that aids surgeons and students in training anytime, anywhere.
A report on global surgery, commissioned in 2015 by The Lancet, found nearly one-third of the global burden of disease can be treated surgically and that five billion people lack access to safe and affordable surgical care.1
“We’re looking to improve the standards of surgical care and treatment around the world, accelerating our pace of innovation and aiding the training of more physicians through collaborations such as this agreement with Touch Surgery,” says Michael del Prado, Company Group Chairman, Ethicon. “It reflects our broad-based approach to innovation and is another important step toward developing a trusted education ecosystem that improves patient outcomes.”
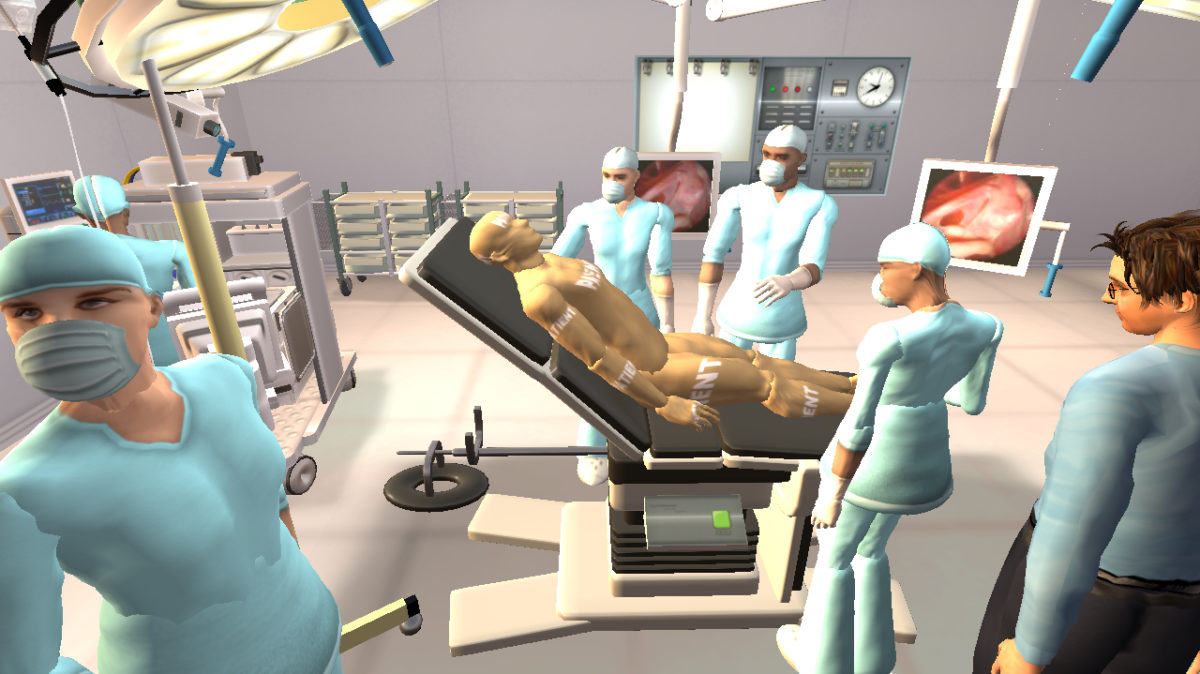
The Touch Surgery platform empowers and connects the global surgery community by enabling virtual surgical training on procedures in 3-D operating room simulations that are unlike other available solutions. The app currently has one million users and helps enable surgeons and other healthcare providers to practice more than 75 procedures of varying surgical specialties.
Ethicon and Johnson & Johnson Medical Device Companies are committed to expanding access to surgical procedures around the world, especially in developing countries where access to the latest training practices can be limited. The collaboration with Touch Surgery will help bolster the library of procedures to create leading educational content across a broad range of surgical specialties, helping to expand resident education and standardize procedures.
Ethicon will begin by addressing training needs in General Surgery and will look at potential opportunities to expand over time to include other Johnson & Johnson Medical Device Companies businesses and specialties, such as orthopaedics and cardiovascular.
About Ethicon
From creating the first sutures, to revolutionizing surgery with minimally invasive procedures, Ethicon has made significant contributions to surgery for more than 60 years. Through Ethicon’s surgical technologies and solutions including sutures, staplers, energy devices, trocars and hemostats and our commitment to treating serious medical conditions like obesity and cancer worldwide, we deliver innovation to make a life-changing impact. Learn more at www.ethicon.com, and follow us on Twitter @Ethicon. Ethicon represents the products and services of Ethicon, Inc. (the signing party) and certain of their affiliates.
About Johnson & Johnson Medical Devices
Having made significant contributions to surgery for more than a century, the Johnson & Johnson Medical Devices Companies are in the business of reaching more patients and restoring more lives. The group represents the most comprehensive surgical technology and specialty solutions business in the world, offering an unparalleled breadth of products, services, programs and research and development capabilities directed at advancing patient care while delivering clinical and economic value to health care systems worldwide.
1The Lancet Commission on Global Surgery, 2015,http://www.who.int/hrh/news/2015/lancet_commission_globsurgery/en/
Cautions Concerning Forward-Looking Statements
This press release contains “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 related to a new collaboration to expand access to digital training in surgical procedures. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Ethicon, Inc., any of the other Johnson & Johnson Medical Devices Companies and/or Johnson & Johnson. Risks and uncertainties include, but are not limited to: the potential that the expected benefits and opportunities related to the collaboration may not be realized or may take longer to realize than expected; uncertainty of success and continued use of the app; competition, including technological advances, new products and patents attained by competitors; changes in behavior and spending patterns of purchasers of health care products and services; and global health care reforms and trends toward health care cost containment. A further list and description of these risks, uncertainties and other factors can be found in Johnson & Johnson’s Annual Report on Form 10-K for the fiscal year ended January 3, 2016, including in Exhibit 99 thereto, and the company’s subsequent filings with the Securities and Exchange Commission. Copies of these filings are available online at www.sec.gov, www.jnj.com or on request from Johnson & Johnson. None of the Johnson & Johnson Medical Devices Companies or Johnson & Johnson undertakes to update any forward-looking statement as a result of new information or future events or developments.
MEDIA CONTACTS:
Peggy Ballman
Mobile: 908-310-7721
pballman@its.jnj.com
SOURCE Ethicon
Related Links
http://www.ethicon.com