Acron Medical Announces FDA 510(k) Clearance of its Signature PEEK TLIF Interbody System, the ACRON Interbody
ORLANDO (PRWEB) JULY 16, 2018
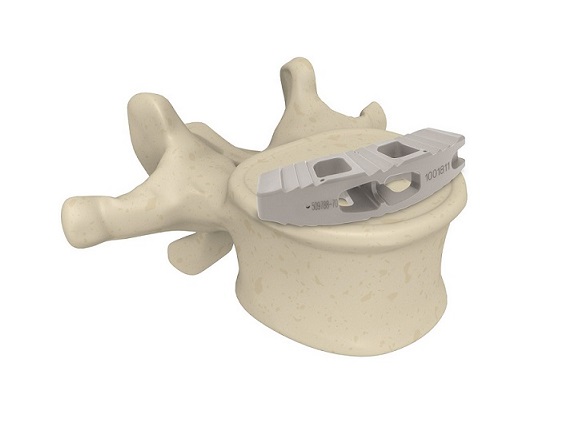
Acron Medical, LLC (http://www.Acron-Medical.com), part of the spineMED Group, is a spine technology organization, dedicated to developing and commercializing globally innovative spinal implants. The company is proud to announce that it has received 510(k) clearance from the FDA for its signature new technology, the ACRON TLIF Interbody system.
“The ACRON Interbody is an exciting innovation for safe & stable thoracolumbar interbody fusion,” says Christian Schawrda, Acron Medical’s Co-Founder and CEO. “The engineering behind the development of the cage makes it the gold standard for surgeons looking for solid anchorage and optimal load distribution between the implant to protect osteoporotic patients from bone subsidence. The cage is designed to be strategically placed along the outer rim of the endplates, or ring apophysis, with superior load-bearing capacity. That approach, maximizes the strength of the fusion construct, increases fusion rates and minimizes the risk of subsidence.”
The ACRON is made of PEEK-OPTIMA, a material with a proven clinical history and a modulus of elasticity similar to bone. It also comes with a graft chamber and side windows to facilitate the formation of a robust fusion column.
The system includes a full range of implant sizes and heights that are carefully designed to accommodate different types of vertebral anatomy, and to allow surgeons to address each patient’s sagittal plane requirements.
Andreas Bernegger, Acron Medical’s Co-Founder says, “The ACRON comes to the United States after 3 years of clinical validation in Austria, Germany and Switzerland. The launch of the technology in the United States is major milestone for our group. We are currently looking for distributors and strategic partners around the country.”
Acron Medical’s management team will be attending NASS in September in Los Angeles (Sep 26-29) and is looking forward to many fruitful discussions with potential partners. For more information on Acron Medical, please visit http://www.Acron-Medical.com.
About Acron Medical, LLC.
Acron Medical is the US subsidiary of the spineMED group, based in Austria. Acron Medical founded in 2017, has leveraged its Austrian DNA, 35 years of clinical experience and the core competencies of the spineMED Group to develop a range of clinical relevant spinal technologies. The ACRON TLIF, as well as all the technologies in the spineMED R&D pipeline, are designed to harmoniously combined the latest scientific advances with intuitive designs and instrumentation. Additional information is available online at http://www.Acron-Medical.com.
OrthoSpineNews
OrthoSpineNews.com is the preferred aggregator of all news in the orthopedic and spine industry. You can subscribe for our daily email, follow us on twitter or download our app for iPhone and Android.